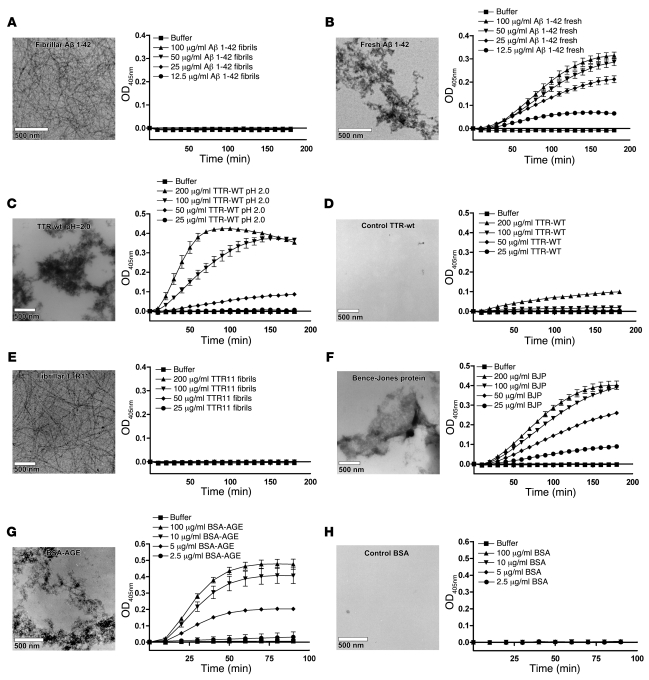
Figure 2. FXII-dependent kallikrein generation is induced by misfolded protein aggregates, but not by amyloid fibrils, in vitro.
FXII-dependent kallikrein generation was measured in vitro by a chromogenic assay. The conversion of 0.3 mM Chromozym PK in the presence of 7.7 nM PK was determined in the presence and absence of 0.97 nM FXII. None of the protein preparations tested was capable of converting Chromozym PK in the presence of PK without FXII present (data not shown). Amyloid fibrils of Aβ 1–42 did not stimulate FXII-dependent kallikrein generation (A), whereas amorphous aggregates of the freshly dissolved peptide did (B). Similarly, only amorphous aggregates of TTR (C), but not native TTR (D), or amyloid fibrils of TTR11 (E) could induce FXII-dependent kallikrein generation. Also, amorphous aggregates of Bence-Jones protein (BJP) (F) and BSA-AGE (G), but not freshly dissolved control BSA (H), could induce FXII-dependent kallikrein generation. The values in the graphs represent the mean ± SEM of duplicate determinations performed within 1 representative experiment of at least 3.