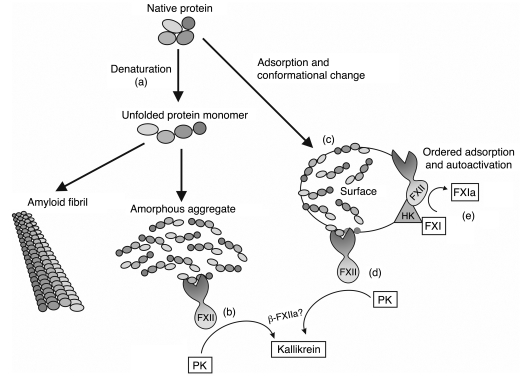
Figure 6. Activation of FXII by misfolded protein aggregates leads to specific activation of the kallikrein-kinin system.
A protein can lose its conformation in a number of ways, most notably by denaturation (a) (e.g., due to mechanical stress). Loss of conformation can lead to exposure of sites that are normally hidden within the protein molecule, e.g., due to electrostatic forces in an aqueous environment. As a result, the unfolded protein molecules will either aggregate into amorphous aggregates that can activate FXII-dependent kallikrein generation in the absence of a surface (b) or, in some cases, assemble into amyloid fibrils that do not activate this system independently. Apparently, amyloid fibrils have lost the epitope(s) required for activation of FXII. During adsorption of proteins to classical surface materials such as kaolin, several proteins also lose their native conformation (c), as was shown for BSA, Fg, and endostatin. These adsorbed proteins are able to stimulate FXII-dependent kallikrein generation (d). Misfolded proteins are not capable of inducing clotting, since they are unable to initiate FXII-dependent FXIa generation (e).