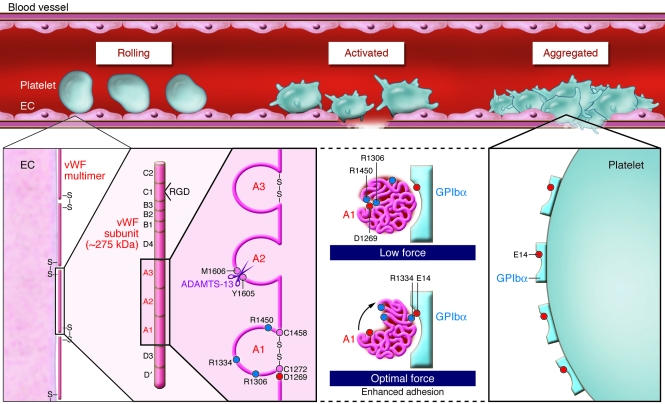
Figure 1. vWF-dependent platelet adhesion at high shear.
GPIb–IX-V–dependent adhesion of human platelets to multimeric vWF on the vessel wall — initiating thrombus formation — involves GPIbα binding vWF A1, a conformationally activated domain of vWF. In their study in this issue of the JCI, Yago et al. (8) show how shear force disrupts vWF A1 electrostatic interactions of D1269 with R1306 and R1450, reorientating vWF A1 relative to receptor and aligning R1334 of vWF to interact with E14 on GPIbα, facilitating adhesion under flow (red and blue circles denote negatively and positively charged residues, respectively; see ref. 8 for structures). The authors show that vWD type 2B mutations (R1306Q or R1450E) constitutively disrupt interactions with D1269, enhancing binding to GPIbα, as well as promoting ADAMTS-13–dependent cleavage within vWF A2. These findings explain how these vWD mutations lead to enhanced vWF binding to platelet GPIbα and depletion of vWF associated with type 2B vWD. In WT vWF, the shear-induced changes in the binding interaction between vWF and GPIbα show how platelet adhesion is enhanced under the influence of shear force and how thrombus formation can be initiated at the vessel wall in flowing blood.