Abstract
Aquaporin-4 (AQP4) is a water-selective transport protein expressed in glial cells throughout the central nervous system. AQP4 deletion in mice produces alterations in several neuroexcitation phenomena, including hearing, vision, epilepsy, and cortical spreading depression. Here, we report defective olfaction and electroolfactogram responses in AQP4-null mice. Immunofluorescence indicated strong AQP4 expression in supportive cells of the nasal olfactory epithelium. The olfactory epithelium in AQP4-null mice had identical appearance, but did not express AQP4, and had ∼12-fold reduced osmotic water permeability. Behavioral analysis showed greatly impaired olfaction in AQP4-null mice, with latency times of 17 ± 0.7 vs. 55 ± 5 s in wild-type vs. AQP4-null mice in a buried food pellet test, which was confirmed using an olfactory maze test. Electroolfactogram voltage responses to multiple odorants were reduced in AQP4-null mice, with maximal responses to triethylamine of 0.80 ± 0.07 vs. 0.28 ± 0.03 mV. Similar olfaction and electroolfactogram defects were found in outbred (CD1) and inbred (C57/bl6) mouse genetic backgrounds. Our results establish AQP4 as a novel determinant of olfaction, the deficiency of which probably impairs extracellular space K+ buffering in the olfactory epithelium.—Lu, D. C., Zhang, H., Zador, Z., Verkman, A. S. Impaired olfaction in mice lacking aquaporin-4 water channels.
Keywords: smell, water permeability, olfactogram
Aquaporin-4 (AQP4) is a water-selective transport protein expressed in plasma membranes of glial cells throughout the brain and spinal cord. Studies in AQP4 knockout mice have implicated the involvement of AQP4 in water movement into and out of the brain (1, 2), in migration of glial cells (3, 4), and in neuroexcitation phenomena. With regard to neuroexcitation, mice lacking AQP4 manifest reduced auditory brainstem responses (5), reduced electroretinogram potentials (6, 7), reduced seizure susceptibility (8), increased seizure duration (9), and slowed dynamics of cortical spreading depression (10). Although not expressed in excitatory cells (neurons in brain, bipolar cells in retina, hair cells in inner ear), AQP4 is expressed in closely associated “supportive” cells, including glial cells in the brain, Müller cells in the retina, and Claudius/Hensen cells in the inner ear. How AQP4 deficiency results in altered neuroexcitation is not known; postulated mechanisms include altered extracellular space K+ handling (11) and volume (12, 13).
AQP4 protein expression has been reported in the rat olfactory system, including basal cells, Bowman’s glands, and olfactory epithelial cells (14, 15). The olfactory epithelium (OE) is a remarkable sensory organ capable of distinguishing thousands of odors at low concentrations (16, 17). Cell bodies of olfactory receptor neurons (ORNs) lie in a pseudostratified epithelium and are protected from the outside environment by an apical layer of supportive sustentacular cells. Each ORN extends a dendrite to the luminal surface, where it terminates in a specialized structure, the dendritic knob. Long, nonmotile olfactory cilia (18), which contain olfactory receptors, extend from the dendritic knob and rest within a mucous layer on the surface of the OE. Basal cells on the basolateral surface of the OE differentiate to produce new ORNs.
Here, based on AQP4 expression in the rat in cell types likely involved in olfaction and the involvement of AQP4 in several nonolfactory neuroexcitatory phenomena, we tested the functional role of AQP4 in olfaction. We found by behavioral analysis greatly impaired olfaction in AQP4-null mice and investigated the cellular basis of this phenotype by determination of OE water permeability, electroolfactogram (EOG) responses, and AQP4 expression pattern.
MATERIALS AND METHODS
Mice
AQP4-null mice in a CD1 (outbred) genetic background were generated at the University of California, San Francisco (UCSF) by targeted gene disruption, as described (19). Mice in a C57/bl6 (inbred) genetic background were generated by >8 back-crosses. Eight- to 12-wk-old weight-matched wild-type and AQP4-null mice were used. Mice were maintained in air-filtered cages and fed normal mouse chow in the UCSF animal care facility. All procedures were approved by the UCSF Committee on Animal Research.
Olfaction behavioral testing
Two independent behavioral tests of olfaction were carried out, with the experimenter blinded to genotype information in all trials. The well-established “buried food pellet” test was used, as described (20, 21). The buried food pellet test, through the use of Purina mouse chow pellets (PMI Nutrition International, Inc., Richmond, IN, USA), eliminated the need for pretraining to associate an exogenous food odor. Mice were placed on a food-restricted diet (0.2 g chow/mouse/24 h) for 3 days before testing and during the 4-day experimental period. Mice had free access to water. Before the first experimental trial, the mice were familiarized with the experimental setting by placement in the enclosure for 10 min daily over the 3 days prior to trials. One trial was conducted daily for each of the 3 testing days. In each trial, a single mouse was placed in a test cage (45×24×20 cm) to recover a 0.5 g food pellet that was buried ∼0.5 cm below the surface of a 3 cm deep layer of mouse bedding material. The location of the pellet was changed daily at random. The latency time was recorded and defined as the time between placement of the mouse in the cage and grasping the food pellet with its forepaws or teeth. Mice were allowed to consume the pellet and then returned to their cage. The bedding in the test chamber was changed between trials. As a control, a visible pellet test was done identically, except that the food pellet was placed randomly on top of the bedding. This control test was carried out on all mice after each daily trial, and mice were allowed to consume the food pellet.
In the “olfaction maze” test, a maze bounded by a circular wall with a 76.2 cm diameter (diagrammed in Fig. 1B) was constructed. Within this circular enclosure were placed fixed, open corners, evenly spaced within the perimeter. The corners allowed odorants such as a food pellet to be hidden from sight but detected by smell. Mice were placed on a food-restricted diet as described above for 3 days before testing and during the 3-day experimental period. Before the first experimental trial, the mice were familiarized with the maze by placement in the maze for 10 min daily over the 3 days prior to trials. For the experimental trial, a mouse was placed at the center of the maze, and a 0.5 g Purina mouse chow pellet was placed at random behind one of the corners. The latency was defined as the time between mouse placement in this maze and discovery and grasping of the food pellet. The mouse was allowed to consume the pellet before returning to its cage. After each trial, the platform was cleaned with 70% ethanol, then water, to remove dirt and odorants. As a control, after each experimental trial, mice were exposed to a visible food pellet in which the 0.5 g pellet was placed at the perimeter of the circular enclosure without the corners.
Figure 1.
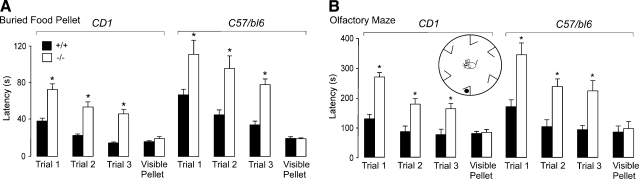
Impaired olfaction in AQP4-null mice shown by behavioral testing. Olfactory performance was tested in buried food pellet and olfactory maze tests as described in Materials and Methods. A) Buried food pellet test. The latency for mice (CD1 and C57/bl6 genetic backgrounds) to recover a buried food pellet was recorded on each of 3 consecutive testing days (mean±se, 10 mice/group; *P<0.001, paired t test). Latency times not significantly different in control visible pellet test (P=0.16 for CD1, P=0.31 for C57/bl6; control data shown from last testing day). B) Olfactory maze test. The latency for mice (CD1 and C57bl6 genetic backgrounds) to recover a hidden food pellet was recorded (mean±se, 10 mice per group; *P<0.001, paired t test). Latency times not significantly different in control visible pellet test (P=0.21 for CD1; P=0.28 for C57/bl6). Inset: schematic of maze.
EOG
The method for EOG preparation was adopted from Scott et al. (22), with minor modifications. EOG measurements were done without the knowledge of mouse genotype. Immediately before electrophysiological recording, mice were anesthetized with intraperitoneal Avertin (125 mg/kg) and decapitated. The nasal septum and midline aspect of the nasal epithelium over the endoturbinate bones were surgically exposed under an operating microscope, and hemisectioning along the sagittal suture was done with a surgical blade. The hemisection was immobilized with moldable clay in a transparent perfusion chamber. The chamber was perfused with humidified air at 100 cc/min constant flow under continuous suction. An injection port in which odorants could be introduced was located near the OE. Teflon™-coated tubing was used to prevent odor contamination. To prevent drying of the mucosal surface 50 μl of PBS was periodically dripped onto the OE. Odorants representing different chemical classes were tested, including C2–8 aldehydes, ketones, esters, alcohols, and carboxylic acids. Saturated vapors from compounds to be tested were drawn into a Teflon-coated glass syringe and 1, 5, 10, or 20 cc of the vapor was injected into the perfusion chamber over 500 ms during EOG recordings.
The method of EOG recording was adapted from Ottoson (23). Recording electrodes with tip diameter ∼1 μm were pulled from borosilicate capillary glass tubing (Sutter Instruments, Novato, CA., USA) and filled with PBS. Tip resistance was ∼3 MΩ. The recording electrode was positioned at the center of the first (most anterior) turbinate, where the consistently greatest responses to odorants were found. The Ag/AgCl reference electrode was placed on the frontal bone near the cribiform plate. Electrodes were connected to a high-gain, high-impedance electroencephalogram amplifier module of a MP100 data acquisition system (Biopac Systems, Inc., Goleta, CA, USA). Signals were low-pass filtered at 30 Hz and digitized at 125 Hz.
Osmotic water permeability measurements
A calcein-quenching method was used in which intracellular calcein fluorescence is quenched instantaneously by cytoplasmic proteins and hence sensitive to cell volume (24). The OE was exposed as described above. Small fragments of the epithelium were isolated and stained with calcein by incubation with 5 μM calcein-AM (Invitrogen, Eugene, OR) for 30 min at 37°C. A perfusion chamber modified from an open bath slice chamber (RC-26; Warner Instruments, Hamden, CT, USA) was positioned on the stage of an inverted epifluorescence microscope (Nikon Diaphot; Nikon, Tokyo, Japan). The OE, apical surface facing down, was stabilized by 2 nylon meshes (1 mm grid spacing; Fig. 2A). The chamber volume was ∼350 μl, giving a measured solution exchange half-time of <400 ms at 50 ml/min perfusion rate. Solutions bathed all surfaces of the epithelium. The time course of cytoplasmic calcein fluorescence was measured at room temperature in response to cell swelling/shrinking produced by exchange of perfusate between PBS and hypotonic PBS (diluted 1:1 with water). Calcein fluorescence was detected using an objective lens (×25, long working distance Fluro, Nikon) focused within the OE. The microscope was equipped with stabilized halogen light source, calcein filter set (480 nm excitation, 490 nm dichroic mirror, 535 nm emission filter), photomultiplier detector, and 14 bit analog-to-digital converter.
Figure 2.
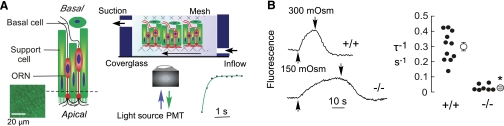
Reduced osmotic water permeability in AQP4-deficient olfactory epithelium. A) Left panel: diagram of the olfactory epithelium. Inset: calcein-stained OE. Right panel: schematic of perfusion chamber with freshly isolated olfactory epithelium immobilized on the nylon mesh and all surfaces exposed to solutions. Inset: perfusate exchange time as measured by perfusion with PBS followed by fluorescein-containing PBS. B) Left panel: representative calcein fluorescence recordings in response to hypoosmolar challenge. Solution osmolarities changed from 300 to 150 mosmol at upward deflections and 150 to 300 mosmol at downward deflections. Right panel: reciprocal exponential time constants for osmotic equilibration (τ−1, in s−1) in olfactory epithelium from wild-type and AQP4-null mice. Individual measurements (•) and mean ± se (○). *P < 0.001.
Immunohistochemistry and immunofluorescence
Mice were anesthetized by intraperitoneal Avertin (125 mg/kg) and perfused transcardially with 4% paraformaldehyde in PBS. The mice were decapitated, and then the nasal cavity was dissected and postfixed for 24 h in paraformaldehyde at 20°C. Tissues were dehydrated with increasing concentrations of ethanol, treated with clearing agent, and embedded in paraffin. Some sections were deparaffinized and stained with hematoxylin and eosin using standard procedures. For immunofluorescence, epitope exposure was enhanced by incubation in citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0; 30 min, 95–100°C). Sections were blocked with bovine serum albumin (3%), incubated with rabbit anti-AQP4 antibody (1:500, Chemicon, Temecula, CA, USA) and goat anti-olfactory marker protein (OMP) antibody (1:500, Wako Chemicals, Richmond, VA, USA) and detected using Texas Red donkey anti-rabbit (1:100; The Jackson Laboratory, Bar Harbor, ME, USA) or FITC donkey anti-goat secondary antibody (1:200, Molecular Probes, Eugene, OR). Immunofluorescence was done similarly on isolated, fixed cells immobilized on coverslips, using rabbit anti-AQP4 antibody (1:500) and goat anti-OMP antibody (1:5000), and detected using Texas Red donkey-anti-rabbit (1:200) or fluorescein isothiocyanate donkey anti-goat secondary antibody (1:200). Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). The OE from nasal turbinates was transferred to Ca2+- and Mg2+-free Hanks’ medium, minced into ∼1 mm fragments, and washed. Cells were enzymatically dissociated by incubation in 0.1% collagenase at 37°C for 5 min. After washing in Ca2+- and Mg2+-free Hanks’ medium, cells (∼104 per coverslip) were immobilized on 12 mm diameter round glass coverslips coated with concanavalin A.
RESULTS
Impaired olfaction in AQP4-null mice
Behavioral analysis of olfaction was performed first using the well-established buried food pellet recovery test, in which the latency is measured for food-deprived mice to find a buried food pellet (20, 21). Figure 1A shows a significantly greater latency time for AQP4-null mice on each of the trial days. The difference was seen in both outbred (CD1) and inbred (C57/bl6) genetic backgrounds. As control, the latency time for recovery of a visible food pellet did not differ significantly in wild-type and AQP4-null mice.
A second set of behavioral olfaction studies was done using an independent method to confirm defective olfaction in AQP4-null mice. As described in Materials and Methods, we devised an olfactory maze test in which the latency is measured for mice to recover a hidden food pellet, as diagrammed in Fig. 1B (inset). Latency times were significantly greater for the AQP4-null mice, in both outbred and inbred genetic backgrounds, in each of the trials (Fig. 1B). No significant difference in latency times was found in the control visible food pellet test. These results establish defective olfaction in AQP4-null mice. To investigate the cellular mechanisms responsible for defective olfaction, we conducted functional studies of water permeability and electrical responses in OE and determined the cellular expression pattern of AQP4.
Reduced water permeability and EOG responses in OE
To determine whether AQP4 in the OE is functional as a plasma membrane water channel, we measured the apparent osmotic water permeability of the OE. A calcein fluorescence quenching method was used in which the kinetics of cytoplasmic calcein fluorescence was recorded in response to externally applied osmotic gradients. Measurements were made on calcein-stained OE using a perfusion chamber designed for rapid solution exchange (Fig. 2A). Because all surfaces of the OE are perfused and subjected to osmotic gradients, this method measures the composite responses of all cell types in the OE, and cannot distinguish individual cell types. The objective lens was focused on a volume in the OE containing both calcein-stained ORNs and supportive cells (Fig. 2A, inset). Figure 2B, left panel, shows the time course of calcein fluorescence in response to changes in perfusate osmolarity between 300 and 150 mosmol. Osmotic equilibration was remarkably slowed in the AQP4-null OE. Averaged osmotic equilibration rates (reciprocal exponential time constants, τ) are summarized in Fig. 2B, right panel, showing significant, ∼12-fold slowing in AQP4-null OE (τ−1 0.30±0.03 s−1, wild type vs. 0.025±0.006 s−1, AQP4 null). These studies indicate functional plasma membrane expression of AQP4 in the OE.
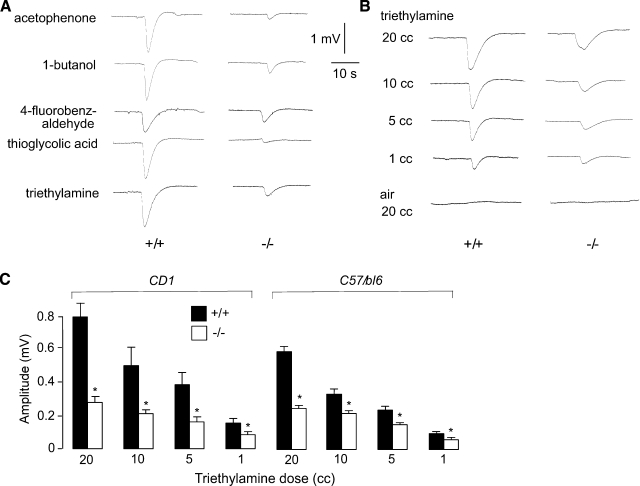
To assess whether the reduced water permeability of the AQP4-null OE results in impaired olfactory signaling, primary olfactory signaling was determined by EOG analysis (23), which measures odorant-induced field potentials across the OE. Compounds from several chemical classes were initially tested on CD1 (Fig. 3A) and C57/bl6 (data not shown) mice, producing similar results. Representative original EOG recordings are shown in Fig. 3A. Though curve shapes were similar, the amplitudes of the voltage responses were lower in AQP4-null OE. No voltage response was seen with application of odorant-free air. Figure 3B shows EOG recordings from wild type and AQP4-null OE in response to increasing doses of triethylamine. Reduced EOG responses were seen in AQP4-null OE for each of the doses. Figure 3C summarizes the voltage response data, showing significantly reduced responses at all triethylamine doses (20, 10, 5, 1 cc) in AQP4-null mice in CD1 and C57/bl6 genetic backgrounds. These results indicate impaired electrophysiological responses to odorants in AQP4 deficiency, accounting for the defective olfaction observed in behavioral studies.
Figure 3.
Impaired electrophysiological olfactory responses in AQP4-null mice. EOGs were recorded in response to brief exposure of the olfactory epithelia to vapor-phase odorants. A) Representative EOGs of wild-type and AQP4-null mice (CD1 genetic background) to odorants of different chemical classes. B) Representative EOGs in response to exposure to air (20 cc) or air saturated with trimethylamine (20, 10, 5, and 1 cc). C) Summary of maximum voltage responses to indicated doses of triethylamine, from experiments as in B, for mice in CD1 and C57/Bl6 genetic backgrounds (mean±se, 10 mice/group, *P<0.005 by paired t test).
AQP4 expression in OE
The studies above indicate functional impairment in olfactory signaling in AQP4-deficient OE. To rule out gross anatomical differences in the OE as accounting for the impaired olfaction, we compared the cellular structure of the OE in wild-type and AQP4-null mice in paraffin sections stained with hematoxylin and eosin. Tissue morphology was indistinguishable between wild-type and AQP4-null mice (data not shown). The cell types in the OE include supportive cells, ORNs, and basal cells lining the basal lamina (25).
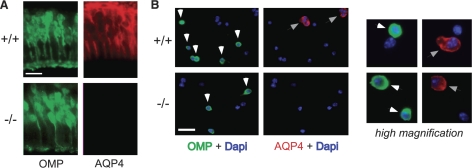
AQP4 immunoreactivity was detected at various locations in the OE, outlining cell membranes (red staining; Fig. 4A). No specific AQP4 immunoreactivity was seen in OE from AQP4-null mice. To assess cell-specific AQP4 expression, the OE was costained with OMP, a marker of mature ORNs (26). OMP-positive ORNs were seen primarily in the basal-facing two-thirds of the OE (green staining; Fig. 4A), where relatively little AQP4 staining was seen, suggesting that ORNs do not express AQP4. However, the proximity of the opposing membranes of neighboring ORNs and supportive cells precluded unambiguous identification of AQP4-expressing cell types.
Figure 4.
AQP4 expression in olfactory epithelium. A) OMP (ORN marker, green) and AQP4 (red) immunofluorescence of OE from wild-type and AQP4-null mouse (apical surface facing down). B) Immunofluorescence of dissociated cells. Left panels: low-magnification images showing green-stained ORNs (white arrows) and red-stained AQP4-expressing cells (gray arrows). Right panels: high-magnification images showing examples of individual cells stained green or red only (top) and one example of a cell stained green and red (bottom). Scale bars = 10 μm (A); 50 μm (B).
Immunostaining was done on separated cells to determine definitively whether ORNs express AQP4. Cells were dissociated by brief enzymatic treatment of the OE and immobilized on glass coverslips coated with concanavalin A, which facilitates ORN attachment (27). ORNs were identified by OMP (green) fluorescence and their characteristic elongated morphology. The left panels in Fig. 4B show low-magnification micrographs. OMP and AQP4 antibodies stained distinct populations of cells, with more than 90% of the OMP-positive ORNs being negative for AQP4. Many OMP-negative cells (supportive cells) expressed AQP4. The right panels in Fig. 4B show high-magnification views of a few cells. The top panels show an ORN (green) next to a supportive cell (red). The bottom panels show one rare example of a green-stained ORN that also expressed AQP4. These results indicate that AQP4 is expressed primarily in supportive cells rather than ORNs.
DISCUSSION
The principle finding of this study is impaired olfaction in AQP4 knockout mice, with reduced osmotic water permeability and odorant-induced excitation in the OE. This work was motivated by AQP4 expression in cell types involved in olfaction and the previously demonstrated neuroexcitation defects in AQP4 deficiency. Our results add to an expanding list of AQP-associated neuroexcitation phenotypes.
We used a previously validated olfactory behavioral test, the buried food pellet test (20, 21) and developed a new olfactory maze test. Whereas other olfactory tests have also been used in mice, including an odor discrimination test (27, 28) and a 2-nozzle drink test (29, 30), these tests require pretraining, and so the results depend not only on olfaction but on other sensory and higher cortical functions. The odor discrimination and 2-nozzle drink tests require learning and memory inputs, and the 2-nozzle drink test examines the sense of taste and its associated pathways (facial and glossopharyngeal nerves) in addition to the sense of smell. Our olfactory maze test primarily examines smell because AQP4-null mice do not manifest motor defects and so can locomote normally to the target food pellet. The olfactory maze test uses a circular enclosure with symmetrical barriers and thus lacks visual cues to further isolate the test to olfaction. We observed improvements in performance with repeated testing in both olfactory behavioral tests, likely the result of learning and conditioning. We found significant impairment in olfaction in AQP4-null mice in both the buried food pellet and olfactory maze tests.
The EOG is a negative electrical potential recorded at the surface of the OE. Odorant binding to apical membrane receptors on ORNs open ion channels, resulting in cell depolarization. The EOG is thus related, in part, to the summated potentials generated by ORNs. Tetradotoxin application to the olfactory surface epithelium did not abolish the EOG (data not shown), which is consistent with previous data (23, 31), suggesting a direct depolarization process involving G-protein/ion channel coupling. Supporting cells also contribute to the EOG signal by retarding the onset and decay kinetics of the EOG signal (32). These nonexcitable supportive cells have strongly negative potentials at rest that change during the odor response (32) and are likely involved in extracellular space K+ buffering (33). It is thus not possible from OE field potential recordings alone to determine the relative importance of defective ORN function and altered OE K+ buffering in AQP4 deficiency.
Similar to other excitatory tissues, such as the retina, inner ear, and cerebral cortex, AQP4 expression is largely confined to the supportive cells of the OE. Costaining of freshly isolated cells from the OE with antibodies against AQP4 and the ORN marker OMP showed AQP4 expression in supportive cells but in very few ORNs. Previous results in rats also showed AQP4 immunostaining in OE (14, 15). AQP4 up-regulation and organization into orthogonal particles in the olfactory mucosa during early development was also shown previously (34). Orthogonal arrays of particles are cobblestone-appearing macromolecular assemblies of AQP4 seen by freeze-fracture electron microscopy. The presence of AQP4 in orthogonal arrays of particles was initially shown in AQP4-transfected cells (35) and proven from their absence in AQP4-null mice (36). The expression of AQP4 in supportive cells of the OE suggests that the defective olfaction in AQP4-null mice is the result of impaired extracellular space K+ buffering.
The ability to smell is critical in mice for suckling in early life (37). Transgenic mice lacking the cyclic nucleotide-gated cation channel are believed to die within the first 2 days of life because they cannot locate the milk source (38). These null mice manifest complete anosmia, with absence of EOG responses when stimulated with odorants. Consistent with their critical role in generating olfactory responses, cyclic nucleotide-gated cation channels were enriched in the cilia in olfactory neurons (39). Our electrophysiological data shows attenuated, albeit intact, EOG responses in AQP4-deficient mice, which translates to functionally impaired olfaction.
It remains unknown how AQP4 deficiency impairs the excitatory function of the OE, as do the mechanisms responsible for altered neuroexcitation in hearing, vision, epilepsy, and cortical spreading depression. Altered extracellular space K+ buffering produced by interactions between AQP4 and the inwardly rectifying K+ channel Kir4.1 has been proposed to account for the neuroexcitation phenotypes in AQP4 deficiency, through recent patch-clamp analysis has provided direct evidence against AQP4-Kir4.1 functional interaction (40, 41). Other K+ or ion channels may be responsible for the delayed reuptake of excess extracellular space K+ after brain neuroexcitation in AQP4-null mice (42) and α-syntrophin null mice, the latter manifesting AQP4 mislocalization (43). Brain extracellular space expansion associated with enhanced solute diffusion was found in AQP4-null mice by cortical surface photobleaching (12) and microfiberoptic photobleaching (13) methods, and proposed by a volume dilution mechanism to contribute to the delayed K+ reuptake. Perhaps reduced water permeability in the OE in AQP4 deficiency, as demonstrated here, may be responsible for defective neuroexcitation function by a mechanism involving impaired cell volume responses. Alternative possible mechanisms include AQP4 interaction with key ion channels in ORNs, perhaps through PDZ-domain interactions, and maladaptive regulation in AQP4 deficiency of other transporters involved in olfaction. Establishing the mechanisms linking AQP4 with neuroexcitation in olfaction presents a significant challenge, as it has in other excitable tissues. Although the data here support defective extracellular space K+ buffering as responsible for defective olfaction in AQP4 deficiency, they do not establish the link between AQP4 and K+ buffering.
Our study demonstrates incomplete anosmia as a new manifestation of AQP4 deficiency. It is not known whether AQP4 deficiency in humans is associated with anosmia. Because deficits in olfaction are not readily identified compared with deficits in other senses, we suspect that deficiency in AQP4 function because of AQP4 polymorphisms, although present, may be difficult to assess without a large clinical trial. Note that many cognitive disorders manifest alterations in olfaction with alterations in AQP4, including Down syndrome (44), multiple sclerosis (45), and schizophrenia (46). It has been suggested that loss of AQP4 expression in response to reactive oxygen species in certain brain regions of Down syndrome may lead to cellular dysfunction and programmed cell death (47), which may occur as well in the OE. Immunoglobulin antibodies against AQP4 are found in a form of multiple sclerosis, neuromyelitis optica (Devic’s syndrome) (48), which may contribute to demyelination in the optic nerve and spinal cord (49). Whether this process occurs in the OE, leading to anosmia, is not known. Last, there is evidence linking the AQP4 gene locus with schizophrenia (50). If our purported mechanism of anosmia is supported in these diseases, it is intriguing to note that the OE, an easily accessible region, may be useful in the diagnosis and therapeutic testing of the aforementioned clinical disorders.
Acknowledgments
We thank L. Qian for mouse breeding and genotype analysis. This work was supported by grants from the U.S. National Institutes of Health (DK35124, EY13574, EB00415, HL59198, DK72517, and HL73856) and Research Development Program and Drug Discovery grants from the Cystic Fibrosis Foundation.
References
- Manley G T, Fujimura M, Ma T, Noshita N, Filiz F, Bollen A W, Chan P, Verkman A S. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- Papadopoulos M C, Saadoun S, Binder D K, Manley G T, Krishna S, Verkman A S. Molecular mechanisms of brain tumor edema. Neuroscience. 2004;129:1011–1020. doi: 10.1016/j.neuroscience.2004.05.044. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos M C, Watanabe H, Yan D, Manley G T, Verkman A S. Involvement of aquaporin-4 in astroglial cell migration and glial scar formation. J Cell Sci. 2005;118:5691–5698. doi: 10.1242/jcs.02680. [DOI] [PubMed] [Google Scholar]
- Auguste K I, Jin S, Uchida K, Yan D, Manley G T, Papadopoulos M C, Verkman A S. Greatly impaired migration of implanted aquaporin-4-deficient astroglial cells in mouse brain toward a site of injury. FASEB J. 2007;21:108–116. doi: 10.1096/fj.06-6848com. [DOI] [PubMed] [Google Scholar]
- Li J, Verkman A S. Impaired hearing in mice lacking aquaporin-4 water channels. J Biol Chem. 2001;276:31233–31237. doi: 10.1074/jbc.M104368200. [DOI] [PubMed] [Google Scholar]
- Li J, Patil R V, Verkman A S. Mildly abnormal retinal function in transgenic mice without Muller cell aquaporin-4 water channels. Investig Ophthalmol Vis Sci. 2002;43:573–579. [PubMed] [Google Scholar]
- Da T, Verkman A S. Aquaporin-4 gene disruption in mice protects against impaired retinal function and cell death after ischemia. Investig Ophthalmol Vis Sci. 2004;45:4477–4483. doi: 10.1167/iovs.04-0940. [DOI] [PubMed] [Google Scholar]
- Binder D K, Oshio K, Ma T, Verkman A S, Manley G T. Increased seizure threshold in mice lacking aquaporin-4 water channels. Neuroreport. 2004;15:259–262. doi: 10.1097/00001756-200402090-00009. [DOI] [PubMed] [Google Scholar]
- Binder D K, Yao X, Zador Z, Sick T J, Verkman A S, Manley G T. Increased seizure duration and slowed potassium kinetics in mice lacking aquaporin-4 water channels. Glia. 2006;53:631–636. doi: 10.1002/glia.20318. [DOI] [PubMed] [Google Scholar]
- Padmawar P, Yao X, Bloch O, Manley G T, Verkman A S. K+ waves in brain cortex visualized using a long-wavelength K+-sensing fluorescent indicator. Nat Methods. 2005;2:825–827. doi: 10.1038/nmeth801. [DOI] [PubMed] [Google Scholar]
- Nagelhus E A, Mathiisen T M, Ottersen O P. Aquaporin-4 in the central nervous system: cellular and subcellular distribution and coexpression with KIR4.1. Neuroscience. 2004;129:905–913. doi: 10.1016/j.neuroscience.2004.08.053. [DOI] [PubMed] [Google Scholar]
- Binder D K, Papadopoulos M C, Haggie P M, Verkman A S. In vivo measurement of brain extracellular space diffusion by cortical surface photobleaching. J Neurosci. 2004;24:8049–8056. doi: 10.1523/JNEUROSCI.2294-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zador Z, Magzoub M, Jin S, Manley G T, Papadopoulos M C, Verkman A S. Microfiberoptic fluorescence photobleaching reveals size-dependent macromolecule diffusion in extracellular space deep in brain. FASEB J. 2007;22:326–332. doi: 10.1096/fj.07-9468com. [DOI] [PubMed] [Google Scholar]
- Sorbo J G, Moe S E, Holen T. Early upregulation in nasal epithelium and strong expression in olfactory bulb glomeruli suggest a role for aquaporin-4 in olfaction. FEBS Lett. 2007;581:4884–4890. doi: 10.1016/j.febslet.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Ablimit A, Matsuzaki T, Tajika Y, Aoki T, Hagiwara H, Takata K. Immunolocalization of water channel aquaporins in the nasal olfactory mucosa. Arch Histol Cytol. 2006;69:1–12. doi: 10.1679/aohc.69.1. [DOI] [PubMed] [Google Scholar]
- Lancet D, Sadovsky E, Seidemann E. Probability model for molecular recognition in biological receptor repertoires: Significance to the olfactory system. Proc Natl Acad Sci U S A. 1993;90:3715–3719. doi: 10.1073/pnas.90.8.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkin R I, Hoye R C. Hyposmia secondary to excision of the olfactory epithelium. The definition of primary and accessory areas of olfaction. Life Sci. 1966;5:331–341. doi: 10.1016/0024-3205(66)90018-x. [DOI] [PubMed] [Google Scholar]
- Getchell M L, Rafols J A, Getchell T V. Histological and histochemical studies of the secretory components of the salamander olfactory mucosa: effects of isoproterenol and olfactory nerve section. Anat Rec. 1984;208:553–565. doi: 10.1002/ar.1092080411. [DOI] [PubMed] [Google Scholar]
- Ma T, Yang B, Gillespie A, Carlson E J, Epstein C J, Verkman A S. Generation and phenotype of a transgenic knockout mouse lacking the mercurial-insensitive water channel aquaporin-4. J Clin Invest. 1997;100:957–962. doi: 10.1172/JCI231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D A, Thompson M L, Burge K G. Olfactory bulb removal vs peripherally induced anosmia: differential effects on the aggressive behavior of male mice. Behav Biol. 1972;7:823–828. doi: 10.1016/s0091-6773(72)80174-3. [DOI] [PubMed] [Google Scholar]
- Nathan B P, Yost J, Litherland M T, Struble R G, Switzer P V. Olfactory function in apoE knockout mice. Behav Brain Res. 2004;150:1–7. doi: 10.1016/S0166-4328(03)00219-5. [DOI] [PubMed] [Google Scholar]
- Scott J W, Davis L M, Shannon D, Kaplan C. Relation of chemical structure to spatial distribution of sensory responses in rat olfactory epithelium. J Neurophysiol. 1996;75:2036–2049. doi: 10.1152/jn.1996.75.5.2036. [DOI] [PubMed] [Google Scholar]
- Ottoson D. Analysis of the electrical activity of the olfactory epithelium. Acta Physiol Scand. 1956;35:122. [PubMed] [Google Scholar]
- Solenov E, Watanabe H, Manley G T, Verkman A S. Sevenfold-reduced osmotic water permeability in primary astrocyte cultures from AQP-4-deficient mice, measured by a fluorescence quenching method. Am J Physiol. 2004;286:C426–C432. doi: 10.1152/ajpcell.00298.2003. [DOI] [PubMed] [Google Scholar]
- Nomura T, Takahashi S, Ushiki T. Cytoarchitecture of the normal rat olfactory epithelium: light and scanning electron microscopic studies. Arch Histol Cytol. 2004;67:159–170. doi: 10.1679/aohc.67.159. [DOI] [PubMed] [Google Scholar]
- Weiler E, Benali A. Olfactory epithelia differentially express neuronal markers. J Neurocytol. 2005;34:217–240. doi: 10.1007/s11068-005-8355-z. [DOI] [PubMed] [Google Scholar]
- Enwere E, Shingo T, Gregg C, Fujikawa H, Ohta S, Weiss S. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci. 2004;24:8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheusi G, Cremer H, McLean H, Chazal G, Vincent J D, Lledo P M. Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc Natl Acad Sci U S A. 2000;97:1823–1828. doi: 10.1073/pnas.97.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien C W, Hisatsune T, Kaminogawa S. Role of olfaction in food preference as evaluated in an animal model. Biosci Biotechnol Biochem. 1999;63:1553–1556. doi: 10.1271/bbb.63.1553. [DOI] [PubMed] [Google Scholar]
- Schaefer M L, Wong S T, Wozniak D F, Muglia L M, Liauw J A, Zhuo M, Nardi A, Hartman R E, Vogt S K, Luedke C E, Storm D R, Muglia L J. Altered stress-induced anxiety in adenylyl cyclase type VIII-deficient mice. J Neurosci. 2000;20:4809–4820. doi: 10.1523/JNEUROSCI.20-13-04809.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker D, Shibuya T. A physiologic and pharmacologic study of olfactory receptors. Cold Spring Harb Symp Quant Biol. 1965;30:207–215. doi: 10.1101/sqb.1965.030.01.023. [DOI] [PubMed] [Google Scholar]
- Trotier D, MacLeod P. Intracellular recordings from salamander olfactory supporting cells. Brain Res. 1986;374:205–211. doi: 10.1016/0006-8993(86)90413-0. [DOI] [PubMed] [Google Scholar]
- Khayari A, Math F, Trotier D. Odorant-evoked potassium changes in the frog olfactory epithelium. Brain Res. 1991;539:1–5. doi: 10.1016/0006-8993(91)90679-p. [DOI] [PubMed] [Google Scholar]
- Rash J E, Davidson K G, Kamasawa N, Yasumura T, Kamasawa M, Zhang C, Michaels R, Restrepo D, Ottersen O P, Olson C O, Nagy J I. Ultrastructural localization of connexins (Cx36, Cx43, Cx45), glutamate receptors and aquaporin-4 in rodent olfactory mucosa, olfactory nerve and olfactory bulb. J Neurocytol. 2005;34:307–341. doi: 10.1007/s11068-005-8360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Brown D, Verkman A S. The mercurial insensitive water channel (AQP-4) forms orthogonal arrays in stably transfected Chinese hamster ovary cells. J Biol Chem. 1996;271:4577–4580. [PubMed] [Google Scholar]
- Verbavatz J M, Ma T, Gobin R, Verkman A S. Absence of orthogonal arrays in kidney, brain and muscle from transgenic knockout mice lacking water channel aquaporin-4. J Cell Sci. 1997;110:2855–2860. doi: 10.1242/jcs.110.22.2855. [DOI] [PubMed] [Google Scholar]
- Teicher M H, Blass E M. First suckling response of the newborn albino rat: the roles of olfaction and amniotic fluid. Science. 1977;198:635–636. doi: 10.1126/science.918660. [DOI] [PubMed] [Google Scholar]
- Brunet L J, Gold G H, Ngai J. General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel. Neuron. 1996;17:681–693. doi: 10.1016/s0896-6273(00)80200-7. [DOI] [PubMed] [Google Scholar]
- Dhallan R S, Yau K W, Schrader K A, Reed R R. Primary structure and functional expression of a cyclic nucleotide-activated channel from olfactory neurons. Nature. 1990;347:184–187. doi: 10.1038/347184a0. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ederra J, Zhang H, Verkman A S. Evidence against functional interaction between aquaporin-4 water channels and Kir4.1 potassium channels in retinal Muller cells. J Biol Chem. 2007;282:21866–21872. doi: 10.1074/jbc.M703236200. [DOI] [PubMed] [Google Scholar]
- Zhang H, Verkman A S. Aquaporin-4 independent Kir4.1 K+ channel function in brain glial cells. Mol Cell Neurosci. 2008;37:1–10. doi: 10.1016/j.mcn.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magzoub M, Padmawar P, Dix J A, Verkman A S. Millisecond association kinetics of K+ with triazacryptand-based K+ indicators measured by fluorescence correlation spectroscopy. J Phys Chem B. 2006;110:21216–21221. doi: 10.1021/jp0633392. [DOI] [PubMed] [Google Scholar]
- Amiry-Moghaddam M, Williamson A, Palomba M, Eid T, de Lanerolle N C, Nagelhus E A, Adams M E, Froehner S C, Agre P, Ottersen O P. Delayed K+ clearance associated with aquaporin-4 mislocalization: phenotypic defects in brains of alpha-syntrophin-null mice. Proc Natl Acad Sci U S A. 2003;100:13615–13620. doi: 10.1073/pnas.2336064100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemdal P, Corwin J, Oster H. Olfactory identification deficits in Down’s syndrome and idiopathic mental retardation. Neuropsychologia. 1993;31:977–984. doi: 10.1016/0028-3932(93)90152-p. [DOI] [PubMed] [Google Scholar]
- Doty R L, Li C, Mannon L J, Yousem D M. Olfactory dysfunction in multiple sclerosis. N Engl J Med. 1997;336:1918–1919. doi: 10.1056/NEJM199706263362617. [DOI] [PubMed] [Google Scholar]
- Hoffer A, Osmond H. Olfactory changes in schizophrenia. Am J Psychiatry. 1962;119:72–75. doi: 10.1176/ajp.119.1.72-a. [DOI] [PubMed] [Google Scholar]
- Esposito G, Imitola J, Lu J, De Filippis D, Scuderi C, Ganesh V S, Folkerth R, Hecht J, Shin S, Iuvone T, Chesnut J, Steardo L, Sheen V. Genomic and functional profiling of human Down syndrome neural progenitors implicates S100B and Aquaporin 4 in cell injury. Hum Mol Genet. 2007 doi: 10.1093/hmg/ddm322. [DOI] [PubMed] [Google Scholar]
- Lennon V A, Kryzer T J, Pittock S J, Verkman A S, Hinson S R. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202:473–477. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemer S F, Parisi J E, Lennon V A, Benarroch E E, Lassmann H, Bruck W, Mandler R N, Weinshenker B G, Pittock S J, Wingerchuk D M, Lucchinetti C F. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain. 2007;130:1194–1205. doi: 10.1093/brain/awl371. [DOI] [PubMed] [Google Scholar]
- Paunio T, Ekelund J, Varilo T, Parker A, Hovatta I, Turunen J A, Rinard K, Foti A, Terwilliger J D, Juvonen H, Suvisaari J, Arajarvi R, Suokas J, Partonen T, Lonnqvist J, Meyer J, Peltonen L. Genome-wide scan in a nationwide study sample of schizophrenia families in Finland reveals susceptibility loci on chromosomes 2q and 5q. Hum Mol Genet. 2001;10:3037–3048. doi: 10.1093/hmg/10.26.3037. [DOI] [PubMed] [Google Scholar]