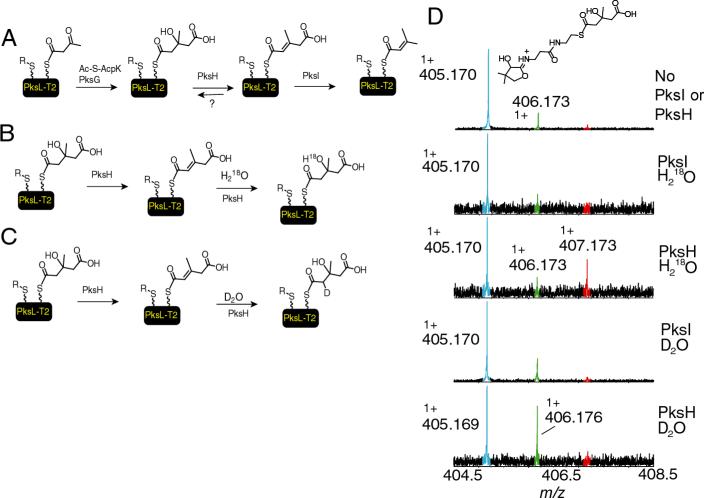
Figure 7.
The rehydratase activity of PksH. A) General overview of the conversion of Acac-S-PksL-2ACP to isoprenyl-S-PksL-2ACP as catalysed by PksG, PksH and PksI. For clarity the chemistry on only one of the two carrier domains is shown. B) Hypothesis for the hydroxyl exchange catalysed by PksH in 70% H218O. C) Hypothesis for the deuterium exchange catlysed by PksH in 70% D2O. D) The data showing the 18O and 2H incorporation into the elimination fragment ion of HMG-S-PksL when the reaction was carried out in 18O or 2H buffers. PksI, served as negative control and demonstrates that the reaction was specific to PksH. The starting HMG-S-PksL-2ACP is generated from racemic HMG-CoA and therefore only 50% of this protein form could serve as a substrate.