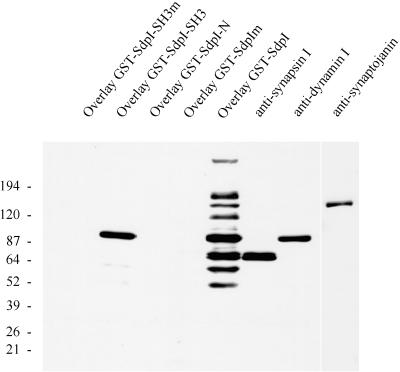
Figure 4.
Overlay analysis of rat brain cytosol with various GST-syndapin I constructs. Rat brain cytosol (20 μg per lane) was separated on 4–15% SDS-PAGE gels, transferred to nitrocellulose membranes, and either overlaid with 1 μM GST fusion proteins of syndapin I (wild-type and P434L mutant of full-length syndapin I and SH3 domain only and SdpI-N, lacking the SH3 domain) or incubated with antibodies against synapsin I, dynamin (2072), and synaptojanin. Binding of GST fusion proteins was detected with affinity-purified anti-GST antibodies. Full-length syndapin I fusion protein (GST-SdpI) reveals major bands at 170 kDa, 145 kDa (comigrating with synaptojanin), 120 kDa, 100 kDa (comigrating with dynamin), 80 and 75 kDa (doublet comigrating with synapsin Ia and Ib), 65 kDa, and 55 kDa, whereas the SH3 domain showed a high preference for the 100-kDa band corresponding to dynamin (GST-SdpI-SH3).