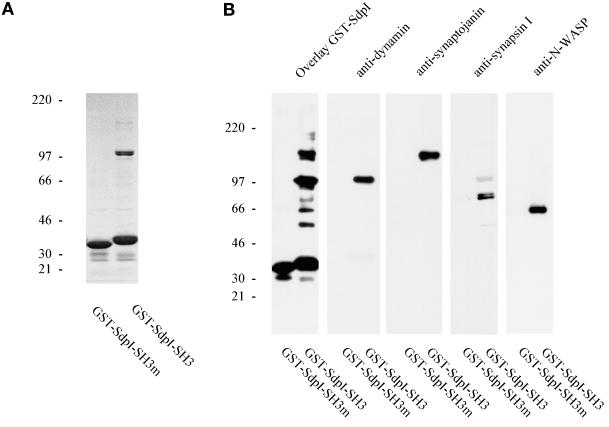
Figure 5.
Identification of major binding partners of syndapin I as dynamin, synaptojanin, synapsin I, and N-WASP. Soluble proteins (S3) from rat brains after homogenization in buffer A were affinity purified onto either wild-type or mutant GST-SdpI-SH3 immobilized on glutathione-Sepharose. Material specifically bound to the beads was eluted with glutathione-containing buffer and analyzed on 4–15% SDS-PAGE. (A) Coomassie protein staining revealed a protein doublet at 100 kDa and a minor band at 145 kDa specifically coprecipitated with wild-type SdpI-SH3 but not with SdpI-SH3m harboring the P434L point mutation. (B) Precipitated material was analyzed by overlay with GST-SdpI and by Western blot analyses. Antibodies against dynamin, synaptojanin, and synapsin I demonstrate that the affinity-purified bands of 100, 145, and 80 and 75 kDa correspond to dynamin, synaptojanin, and synapsin Ia and Ib, respectively. N-WASP–specific antibodies revealed that syndapin I coprecipitated N-WASP because of an SH3 domain-dependent interaction.