Abstract
Background. Intraoperative blood loss has been shown to be an important factor correlating with morbidity and mortality in liver surgery. In spite of the technological advances in hepatic parenchymal transection devices, bleeding remains the single most important complication of liver surgery. The role of radiofrequency (RF) in liver surgery has been expanded from tumour ablation to major hepatic resections in the last decade. HabibTM 4X, a new bipolar RF device designed specifically for liver resection is described here. Methods. HabibTM 4X is a bipolar, handheld, disposable RF device and consists of two pairs of opposing electrodes which is introduced perpendicularly into the liver, along the intended transection line. It produces controlled RF energy between the electrodes and the heat produced seals even major biliary and blood vessels and enables resection of the liver parenchyma with a scalpel without blood loss or biliary leak. Results. Three hundred and eleven patients underwent 384 liver resections from January 2002 to October 2007 with this device. There were 109 major resections and none of the patients had vascular inflow occlusion (Pringle's manoeuvre). Mean intraoperative blood loss was 305 ml (range 0–4300) ml, with less than 5% (n=18) rate of transfusion. Conclusion. HabibTM 4X is an additional device for hepatobiliary surgeons to perform liver resections with minimal blood loss and low morbidity and mortality rates.
Keywords: liver resection, radiofrequency, blood loss, parenchymal transection device, Habib 4X
Introduction
Liver resection has been increasingly performed over the last two decades because of improved postoperative outcomes and evidence that this approach offers the only chance of cure in many patients 1,2,3. Bleeding remains a significant complication of liver resection with 20–60% of patients requiring blood transfusion 4,5. Technical innovations have mainly focused on minimising bleeding during transection of the hepatic parenchyma 4,6 because excessive haemorrhage and blood transfusion have been shown to affect postoperative morbidity, mortality and long-term survival 7,8,9,10.
With improvement in surgical and anaesthetic techniques liver resection can now be performed in most centers with minimal blood loss 11,12,13,14,15,16. However, these techniques often require intraoperative manoeuvres like hypotensive anaesthetics, hepatic pedicle clamping, and total vascular exclusion 11,17,18 to minimise the risk of intraoperative bleeding. None of these methods is completely effective, and each adds a potential risk of causing liver dysfunction in patients with chronic liver disease 19.
In recent years, new surgical tools such as Cavitron ultrasonic aspirator (CUSA), harmonic scalpel, bipolar scissors, Ligasure diathermy or Monoplolar floating ball, have been developed to reduce blood loss during transection of liver parenchyma without vascular clamping 14,15,16. The primary problem relating to these methods is that small vessels can be coagulated during transection, while large vessels are usually left intact or injured, which can result in considerable blood loss during the operation and requires tedious clipping and tying to achieve haemostasis.
In the last decade, the advent of new energy sources, such as radiofrequency (RF), has had an increasing impact on surgical practice, notably in the field of liver tumours. RF was used initially for the local destruction of unresectable liver and other solid organ tumours, applied percutaneously, laparoscopically, or by the open surgical approach. In the recent years, the role of RF in liver surgery has been expanded to routine hepatic resection by using the RF probe to develop a plane of coagulative necrosis along the intended line of parenchymal transection 20,21,22,23. These resections were done using monopolar RF devices primarily designed for tumour ablation, which released uncontrolled amount of energy and produced excessive amount of dead tissue with potential risk of septic complications. To maximise the potential benefits of RF assisted resection a new bipolar RF device (HabibTM 4X, Generator 1500X, RITA Medical Systems, Inc. California, USA) has been developed in our unit and is designed specifically for liver resection. This bipolar RF device releases controlled RF energy between two pairs of electrodes producing a plane of coagulative necrosis along the intended line of parenchymal transection. Here we have described the technical aspect of HabibTM 4X and operative technique for liver resection using this device.
Device description
HabibTM 4X is a bipolar, handheld, disposable RF device and consists of two pairs of opposing electrodes with an active end of 6 or 10 cm in length (Figure 1). The device is connected to a 500-kHz generator (Model 1500X Rita Medical Systems, Inc. California, USA) which produces up to 250 W of RF power. It allows measurement of the generator output, tissue impedance, temperature and time. The system also consists of a pneumatic foot pedal used to turn the RF energy on and off. The generator can be run in manual or automatic mode. On connecting the device and switching on the generator, the RF power setting defaults to 125W and this can be modified according to user experience and the thermal requirement of the individual tissue types.
Figure 1. .
Habib™ 4X – bipolar, handheld, disposable RF device.
Operative technique
After laparotomy, a careful search was performed for local recurrence, extra hepatic and peritoneal disease. Any suspicious lesions were examined with biopsy. Intraoperative bimanual liver palpation and ultrasonography were performed to confirm tumour location and size in all patients. Extensive liver mobilisation was avoided, and only the side to be resected was mobilised in most of the cases. Cholecystectomy was not performed except in cases where the transection line was going through the gall bladder bed. Hilar dissection was not a routine practice, and vascular clamping was not done in any of our cases. Hilar dissection was done only when the tumour was located in close proximity (1 cm or less) to the hepatic hilum. In these cases, separation of the tumour from the hilar structures was achieved by dissection and ligation of the ipsilateral arterial and portal vessels prior to application of the probe to avoid injury to the contralateral hilar structures. Dissection of the hepatic veins was not done routinely unless the tumour was located in close proximity to it, and in such cases dissection and ligation of the hepatic vein was completed before application of the probe to avoid injury to inferior venacava (IVC).
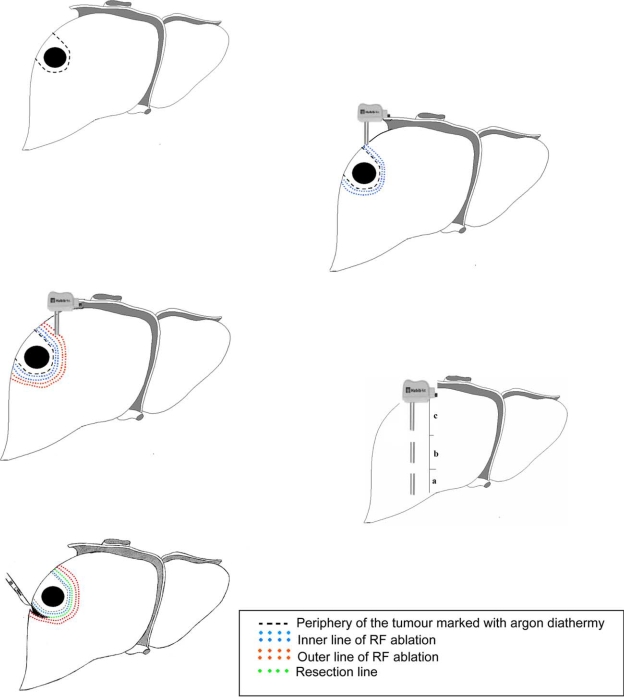
After the tumour was localised the resection line was marked on the liver surface, 1 cm away from the edge of the tumour with an argon diathermy. The marking of the transection line prior to resection is very important, as after RF application the hepatic parenchyma undergoes coagulative necrosis and hardens, which makes palpation of the tumour edge uncertain as well as interferes with intraoperative ultrasound (IOUS) imaging. The HabibTM 4X is then introduced perpendicularly into the liver, abutting the transection line. The generator is programmed to produce an alert signal when energy delivery has been automatically stopped, to avoid over coagulation. This allows a small, less than 10 mm, margin of coagulated liver parenchyma to remain behind ensuring sealed vessels and bile ducts. The process takes less than a minute for each application in the normal liver tissue. The probe is introduced again adjacent to the last coagulated area, in a serial fashion, until the area to be transected is ablated. The number of applications required to create a zone of desiccation is related to the dimensions of the cut surface. Preferably, a second line of ablation parallel to the first line is done to ensure complete tissue coagulation and perfect haemostasis prior to transection (Figure 2).
Figure 2. .
The steps to achieve liver resection using HabibTM 4X.
The surgeon can either apply energy to the whole resection margin and then cut, or apply energy to a partial section and then cut that section, before applying energy to the next section. To coagulate the deep parts of the liver close to the IVC, the probe is introduced parallel to the IVC, and is directed away from it. In tumours located near the liver hilum, after dissection and ligation of the hilar structures the probe is applied parallel to these structures and directed away from hilum. The power of the generator is set at 100 watts for hepatic parenchymal coagulation. For sealing bigger vessels such as hepatic veins, the power is lowered to 80 or even 50 watts. Decreasing power setting achieves a wider coagulation effect in a paradoxical manner. Next, a scalpel is applied perpendicularly to divide the parenchyma near to the first coagulated edge next to the tumour, leaving <10 mm of the coagulated liver parenchyma. During transection, applying a mild compression to the liver parenchyma allows the generated heat to seal the vessels which contributes to a bloodless operative field. For deep tumours, the probe is introduced deep in to the liver parenchyma guided by the surgeon's hand from below. After coagulation of the deep part, the probe is withdrawn in a controlled manner to allow subsequent coagulation of the more superficial parts. The four needles of the probe must be kept parallel to each other by positioning the pushing plate between them to prevent them from converging inside the liver parenchyma and touching each other which can lead to short circuit.
Results
All the liver resections in our institution, ranging from single tumorectomy to major hepatectomies are currently performed using the HabibTM 4X. Three hundred and eleven patients underwent 384 liver resections from January 2002 to October 2007 with this device. The clinical details of these patients are summarised in Table I. There were 109 major resections, which included 84 right hepatectomies, six extended right hepatectomies and 19 left hepatectomies. None of the patients had vascular inflow occlusion (Pringle's manoeuvre). Mean intraoperative blood loss was 305 ml (range 0–4300 ml), with less than 5% (n=18) rate of transfusion. The mean duration of operation was 246 min (range 75–780 min). 16 (4%) patients were admitted to the intensive care unit (ITU) postoperatively. The overall postoperative complication was 21% (n=81). Of these 42 (10%) patients developed intra-abdominal collections, while there were only six (1.6%) biliary leaks. The 30 day mortality was 3.4% (n=13), with no intraoperative deaths during this period. The overall mean postoperative stay was 11.8 days (range 2–65 days).
Table I. Patients’ characteristics and diagnosis.
| Clinical details | |
|---|---|
| Mean age, years | 59±12 |
| Gender (M/F) | 160/151 |
| Diagnosis | |
| Colorectal metastases | 228 (59%) |
| Neuroendocrine metastases | 15 (4%) |
| Non Colorectal Non Neuroendocrine | 52 (14%) |
| Hepatocellular carcinoma | 30 (8%) |
| Cholangicarcinoma | 23 (6%) |
| Gallbladder carcinoma | 5 (1%) |
| Benign | 31 (8%) |
Discussion
Liver resection can be performed with practically no blood loss with this new bipolar RF device without hepatic pedicle clamping, total vascular exclusion or the use of hypotensive anaesthetics. Intraoperative haemorrhage and blood transfusion significantly influence the morbidity and mortality associated with liver surgery 8,11. The transfusion rate of less than 5% in our series is lower than most other hepatic parenchymal transection techniques published in the literature 8,9,11,12,15,16,24,25,26.
HabibTM 4X has allowed us to perform more minor or non-anatomical resections without hepatic pedicle clamping and with minimal blood loss reducing the need for major hepatectomies. Non-anatomical resections have the advantage of sparing liver parenchyma, however they are technically more demanding than a classical lobar resection 9.
Avoidance of hepatic pedicle clamping can prevent ischemia–reperfusion injury to the liver, which is known to predispose to postoperative liver failure 7,27,28,29,30. All our resections were performed without applying Pringle's manoeuvre and therefore the rate of postoperative liver failure in our series was low.
RF energy produces frictional heat caused by intracellular ionic agitation, which leads to coagulative necrosis due to water vaporisation. This bipolar RF device produces a controlled energy flow between the electrodes avoiding over coagulation of liver parenchyma unlike the monopolar devices. The heat produced seals even major biliary and blood vessels and enables resection of the liver parenchyma with a scalpel without blood loss or biliary leak. The amount of energy delivered can be adjusted and is based on the demand for hemostasis. Lower power increases the amount of the coagulated tissue and allows more effective hemostasis as the area of coagulation is broader, whereas high power speeds up the coagulation process due to the narrower coagulation zone. To avoid leaving too much coagulation tissue behind, <1 cm of is left at the resection margin, which is sufficient to achieve vascular and biliary occlusion for hemostasis and biliary control. It is important to mark the resection line with diathermy prior to application of HabibTM 4X as RF produces hardness of liver tissue making the tumour margin difficult to palpate and also increases the echogenicity of IOUS images making it difficult to visualise the tumour edge.
In our experience, no patient had postoperative haemorrhage and the rate of biliary leak was also low at 1.6%, which indicates that biliary control as well as blood vessel occlusion was effective with this device. Apart from achieving a low rate of resection specific complications, the rate of overall postoperative complication in this series was only 21% and is consistent with or better than most reported large series ranging from 16 to 45% 8,9,11,16,24,25,26.
Although this bipolar RF device distributes the RF energy only between the electrodes, its application close to the hilum and the inferior vena cava requires experience, and familiarity with the device. It is essential to dissect the hepatic hilum and the hepatic veins before applying this device close to these structures. This necessitates a detailed knowledge of liver anatomy and good surgical skills while using this device. Major hepatectomies and minor or non-anatomical resections can be performed with this device without the need for ITU admissions, with minimal blood loss and with reduced hospital stay and overall cost of liver surgery. HabibTM 4X is an additional device for hepatobiliary surgeons to perform liver resections with minimal blood loss and low morbidity and mortality rates.
References
- 1.Belghiti J., Hiramatsu K., Benoist S., Massault P., Sauvanet A., Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. doi: 10.1016/s1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 2.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg 2002;236:397–406; discussion 6–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg 2004;240:698–708; discussion 8–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunningham JD, Fong Y, Shriver C, Melendez J, Marx WL, Blumgart LH. One hundred consecutive hepatic resections. Blood loss, transfusion, and operative technique. Arch Surg. 1994;129:1050–6. doi: 10.1001/archsurg.1994.01420340064011. [DOI] [PubMed] [Google Scholar]
- 5.Arnoletti JP, Brodsky J. Reduction of transfusion requirements during major hepatic resection for metastatic disease. Surgery. 1999;125:166–71. [PubMed] [Google Scholar]
- 6.Sitzmann JV, Greene PS. Perioperative predictors of morbidity following hepatic resection for neoplasm. A multivariate analysis of a single surgeon experience with 105 patients. Ann Surg. 1994;219:13–7. doi: 10.1097/00000658-199401000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bismuth H, Castaing D, Garden OJ. Major hepatic resection under total vascular exclusion. Ann Surg. 1989;210:13–9. doi: 10.1097/00000658-198907000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makuuchi M, Takayama T, Gunven P, Kosuge T, Yamazaki S, Hasegawa H. Restrictive versus liberal blood transfusion policy for hepatectomies in cirrhotic patients. World J Surg. 1989;13:644–8. doi: 10.1007/BF01658893. [DOI] [PubMed] [Google Scholar]
- 9.Gozzetti G, Mazziotti A, Grazi GL, Jovine E, Gallucci A, Gruttadauria S, et al. Liver resection without blood transfusion. Br J Surg. 1995;82:1105–10. doi: 10.1002/bjs.1800820833. [DOI] [PubMed] [Google Scholar]
- 10.Kooby DA, Stockman J, Ben-Porat L, Gonen M, Jarnagin WR, Dematteo RP, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg 2003;237:860–9; discussion 69–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Man K, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg 1997;226:704–11; discussion 11–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones RM, Moulton CE, Hardy KJ. Central venous pressure and its effect on blood loss during liver resection. Br J Surg. 1998;85:1058–60. doi: 10.1046/j.1365-2168.1998.00795.x. [DOI] [PubMed] [Google Scholar]
- 13.Sugo H, Mikami Y, Matsumoto F, Tsumura H, Watanabe Y, Kojima K. Hepatic resection using the harmonic scalpel. Surg Today. 2000;30:959–62. doi: 10.1007/s005950070055. [DOI] [PubMed] [Google Scholar]
- 14.Takayama T, Makuuchi M, Kubota K, Harihara Y, Hui AM, Sano K, et al. Randomized comparison of ultrasonic vs clamp transection of the liver. Arch Surg. 2001;136:922–8. doi: 10.1001/archsurg.136.8.922. [DOI] [PubMed] [Google Scholar]
- 15.Sakamoto Y, Yamamoto J, Kokudo N, Seki M, Kosuge T, Yamaguchi T, et al. Bloodless liver resection using the monopolar floating ball plus ligasure diathermy: preliminary results of 16 liver resections. World J Surg. 2004;28:166–72. doi: 10.1007/s00268-003-7167-5. [DOI] [PubMed] [Google Scholar]
- 16.Romano F, Franciosi C, Caprotti R, Uggeri F. Hepatic surgery using the Ligasure vessel sealing system. World J Surg. 2005;29:110–2. doi: 10.1007/s00268-004-7541-y. [DOI] [PubMed] [Google Scholar]
- 17.Belghiti J, Noun R, Malafosse R, Jagot P, Sauvanet A, Pierangeli F, et al. Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Ann Surg. 1999;229:369–75. doi: 10.1097/00000658-199903000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen PD, Isla AM, Habib NA. Liver resection using total vascular exclusion, scalpel division of the parenchyma, and a simple compression technique for hemostasis and biliary control. J Gastrointest Surg. 1999;3:537–42. doi: 10.1016/s1091-255x(99)80109-7. [DOI] [PubMed] [Google Scholar]
- 19.Farges O, Malassagne B, Flejou JF, Balzan S, Sauvanet A, Belghiti J. Risk of major liver resection in patients with underlying chronic liver disease: a reappraisal. Ann Surg. 1999;229:210–5. doi: 10.1097/00000658-199902000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber JC, Navarra G, Jiao LR, Nicholls JP, Jensen SL, Habib NA. New technique for liver resection using heat coagulative necrosis. Ann Surg. 2002;236:560–3. doi: 10.1097/00000658-200211000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Navarra G, Ayav A, Weber JC, Jensen SL, Smadga C, Nicholls JP, et al. Short- and-long term results of intraoperative radiofrequency ablation of liver metastases. Int J Colorectal Dis. 2005;20:521–8. doi: 10.1007/s00384-005-0743-4. [DOI] [PubMed] [Google Scholar]
- 22.Ayav A, Jiao LR, Habib NA. Bloodless liver resection using radiofrequency energy. Dig Surg. 2007;24:314–7. doi: 10.1159/000103664. [DOI] [PubMed] [Google Scholar]
- 23.Ayav A, Bachellier P, Habib NA, Pellicci R., Tierris J., Milicevic M, et al. Impact of radiofrequency assisted hepatectomy for reduction of transfusion requirements. Am J Surg. 2007;193:143–8. doi: 10.1016/j.amjsurg.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Adam R, Pascal G, Azoulay D, Tanaka K, Castaing D, Bismuth H. Liver resection for colorectal metastases: the third hepatectomy. Ann Surg 2003;238:871–83; discussion 83–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber JC, Bachellier P, Oussoultzoglou E, Jaeck D. Simultaneous resection of colorectal primary tumour and synchronous liver metastases. Br J Surg. 2003;90:956–62. doi: 10.1002/bjs.4132. [DOI] [PubMed] [Google Scholar]
- 26.Scatton O, Massault PP, Dousset B, Houssin D, Bernard D, Terris B, Soubrane O. Major liver resection without clamping: a prospective reappraisal in the era of modern surgical tools. J Am Coll Surg. 2004;199:702–8. doi: 10.1016/j.jamcollsurg.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 27.Huguet C, Nordlinger B, Galopin JJ, Bloch P, Gallot D. Normothermic hepatic vascular exclusion for extensive hepatectomy. Surg Gynecol Obstet. 1978;147:689–93. [PubMed] [Google Scholar]
- 28.Delva E, Camus Y, Nordlinger B, Hannoun L, Parc R, Deriaz H, et al. Vascular occlusions for liver resections. Operative management and tolerance to hepatic ischemia: 142 cases. Ann Surg. 1989;209:211–8. doi: 10.1097/00000658-198902000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huguet C, Gavelli A, Bona S. Hepatic resection with ischemia of the liver exceeding one hour. J Am Coll Surg. 1994;178:454–8. [PubMed] [Google Scholar]
- 30.Elias D, Lasser P, Debaene B, Doidy L, Billard V, Spencer A, Leclercq B. Intermittent vascular exclusion of the liver (without vena cava clamping) during major hepatectomy. Br J Surg. 1995;82:1535–9. doi: 10.1002/bjs.1800821126. [DOI] [PubMed] [Google Scholar]