Figure 2.
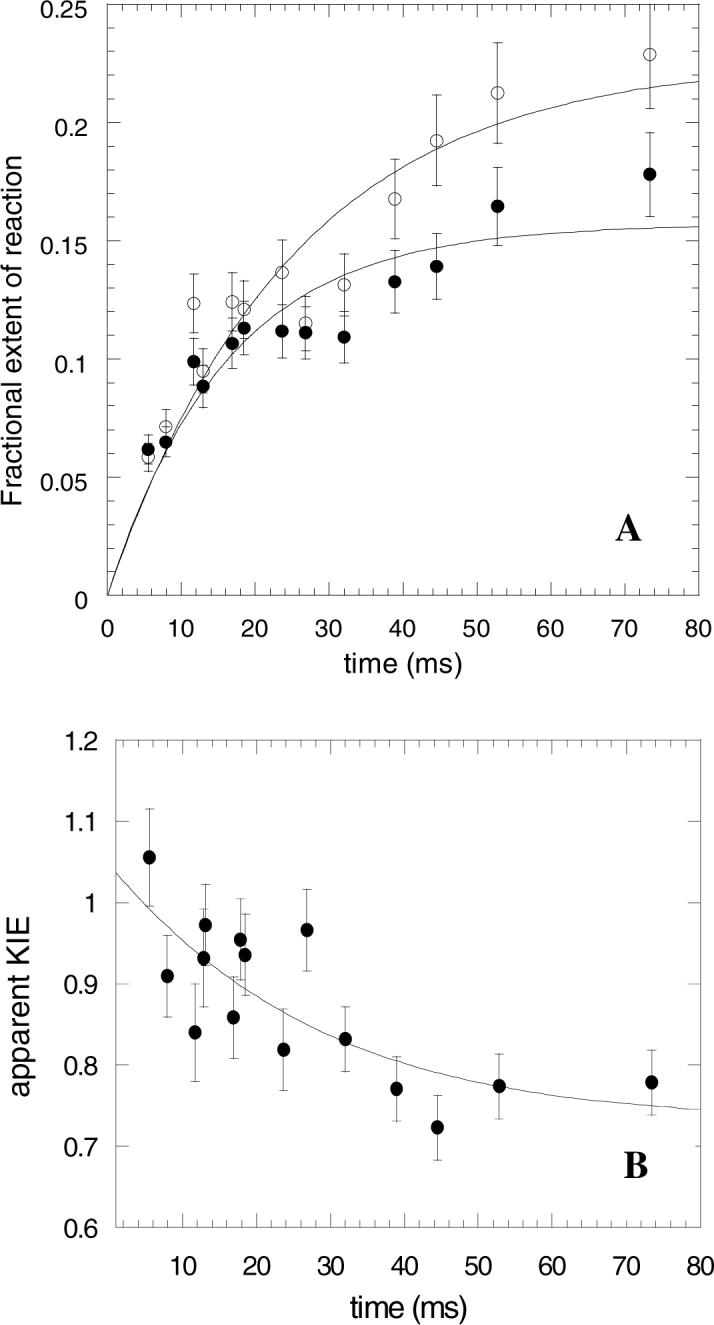
A Kinetics of 5’-dA formation (approach to internal equilibrium) for holo-glutamate mutase reacting with d5-L-glutamate (10 mM). (•) Formation of [5’-H]-dA, as determined by 14C counts; (o) formation of [5’-3H]-dA, as determined by tritium counts. Each time point represents an average of three independent measurements. B Variation of the apparent α-secondary tritium kinetic isotope effect on 5’-dA formation as a function of time, showing the fit of the data to Equation 2..
