Figure 4.
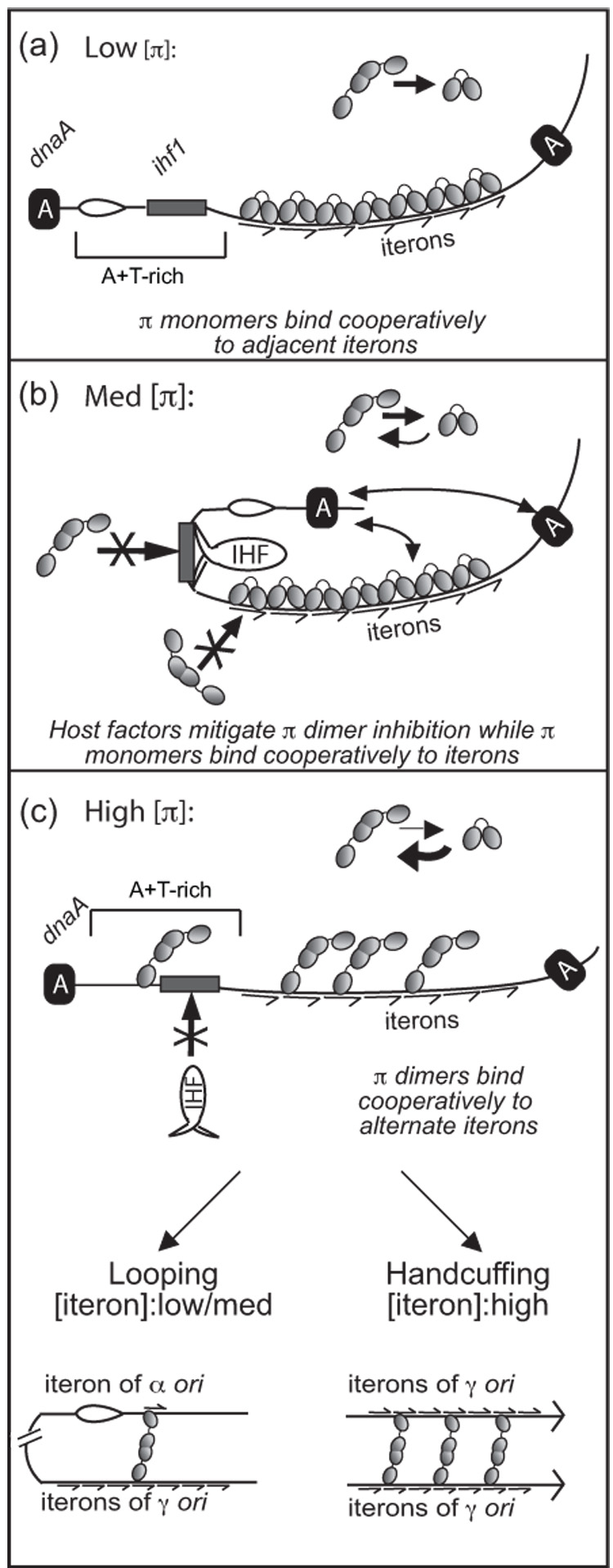
Proposed role of the disparate cooperative binding properties of π monomers and dimers in the regulation of γ ori replication. Symbols representing monomers and dimers of π are the same as in Figure 1. Iterons are depicted by half-arrows. DnaA is labeled as ‘A’. Full arrows represent contacts between different molecules of DnaA and between DnaA and π. The open complex is shown as a bubble. (a), (b), and (c) represent a model for the interactions that occur between π, iteron DNA, and host factors at low, medium, and high levels of intracellular π, respectively. In (a), the host factors IHF and DnaA are not needed at low concentrations of π and monomers of π bind cooperatively to all seven iterons, facilitating γ ori activation. In (b), as π concentration increases, IHF and DnaA help to mitigate the inhibitory effect of πdimers, allowing monomers of π to bind cooperatively to all seven iterons and facilitate γ ori activation. In (c), at high π concentrations, the dimer out-competes IHF for the ihf1 site and occupies alternate iterons of γ ori. The result may be intramolecular looping or intermolecular handcuffing, depending on the concentration of iteron DNA. This model is explained in more depth in the Discussion section.
