Abstract
Levetiracetam, the α-ethyl analogue of the nootropic piracetam, is a widely used antiepileptic drug (AED) that provides protection against partial seizures and is also effective in the treatment of primary generalized seizure syndromes including juvenile myoclonic epilepsy. Levetiracetam was discovered in 1992 through screening in audiogenic seizure susceptible mice and, 3 years later, was reported to exhibit saturable, stereospecific binding in brain to a ∼90 kDa protein, later identified as the ubiquitous synaptic vesicle glycoprotein SV2A. A large-scale screening effort to optimize binding affinity identified the 4-n-propyl analogue, brivaracetam, as having greater potency and a broadened spectrum of activity in animal seizure models. Recent phase II clinical trials demonstrating that brivaracetam is efficacious and well tolerated in the treatment of partial onset seizures have validated the strategy of the discovery programme. Brivaracetam is among the first clinically effective AEDs to be discovered by optimization of pharmacodynamic activity at a molecular target.
Keywords: antiepileptic drug, γ-butyrolactam, piracetam, levetiractam, brivaracetam, audiogenic seizures, kindling, SV2A, rational drug discovery
The discovery of drugs useful in the prevention of epileptic seizures has been a triumph for medicinal chemistry and pharmacology. Drug treatments for epilepsy have been available since 1857, when bromides salts were recognized as having antiseizure activity. In the first half of the twentieth century, two drugs—phenobarbital and phenytoin—became available and they revolutionized the care of persons with epilepsy. Phenobarbital was an early success of synthetic organic chemistry and phenytoin demonstrated the utility of animal models for antiepileptic drug (AED) discovery. Between 1946 and 1978, 16 new AEDs were introduced. A second wave of new drug introductions, now numbering 10, began in 1993 and is currently underway. Most of these new drugs have unique pharmacodynamic properties and they have provided improvements in safety, tolerability and pharmacokinetics, although there is little evidence that they are more efficacious at reducing seizure frequency than older drugs or that they allow previously refractory patients to achieve seizure freedom. Kohn (1989) observed, ‘Nearly all the great discoveries in chemotherapy have been made as a result of a false hypothesis or due to a so-called chance observation.' Indeed, serendipity has played a key role in the discovery of most of the new AEDs, but a critical element has been the availability of predictive animal models. The molecular targets of the AEDs discovered by empirical screening—to the extent that they are known—were usually identified years after the drug had reached the market. For only two marketed agents (tiagabine and vigabatrin) can it be reasonably argued that the drugs were discovered because a specific molecular target was considered.
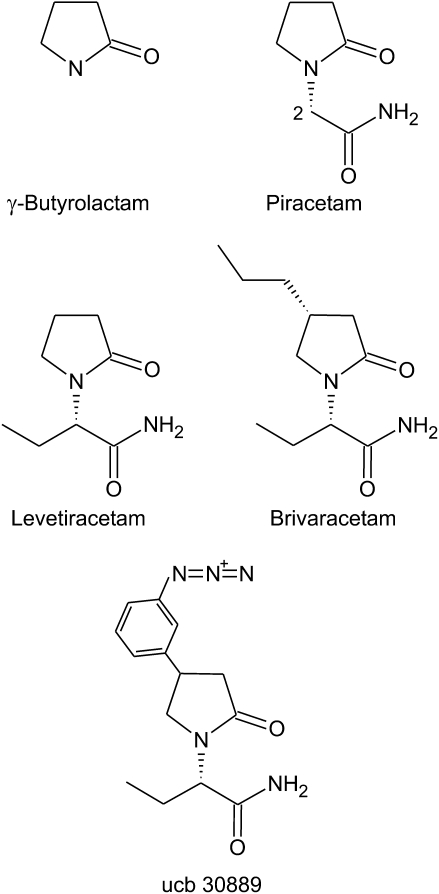
Brivaracetam, an AED currently in late stage clinical development, represents an unusual success of rational drug discovery, in that the discovery process began with a specific molecular target. However, like most other AEDs, brivaracetam exists because of false hypotheses and chance observations. In the early 1960s, in a search for new sedative hypnotic agents, several cyclic GABA analogues were synthesized based on γ-butyrolactam (2-oxopyrrolidine) (Figure 1). The view was that these agents would calm the brain by interacting with GABA systems. However, instead of being tranquilizers, some of these analogues, including the 2-acetamide piracetam, were found to improve memory in rodents. The mechanism of this effect is still unclear, but it is certainly not related to GABA. In any case, piracetam is prescribed in the belief that it has beneficial effects on memory and cognitive function. Studies in animal models have demonstrated that piracetam has weak anticonvulsant actions (Löscher and Hönack, 1993), but the drug is not used clinically for the treatment of epilepsy, although it is approved in some markets for myoclonus.
Figure 1.
2-Oxopyrrolidine (γ-butyrolactam) and analogues.
In 1992, Alma Gower, working at UCB, observed in routine screening that the (S)-enantiomer of the ethyl analogue of piracetam provided more potent protection against sound-induced convulsions in audiogenic seizure susceptible mice than piracetam (Gower et al., 1992). This compound—known today as levetiracetam—had activity in a variety of other seizure models, although its spectrum of activity was distinctly different from other AEDs (Löscher and Hönack, 1993). Importantly, levetiracetam failed to confer protection in the two most widely used AED screening models, in which all other clinically used AEDs show activity that employ a supramaximal electrical (maximal electroshock test) or chemical (subcutaneous pentylenetetrazol test) stimulus (Klitgaard et al., 1998). However, levetiracetam suppresses spike-wave discharges in absence seizure models (Gower et al., 1995), and there is now clinical evidence that it is efficacious in the treatment of absence epilepsy in humans and other forms of primary generalized epilepsy, including juvenile myoclonic epilepsy. In contrast to the activity of the (S)-isomer, the (R)-form of levetiracetam was at least 150-fold less potent in the audiogenic seizure susceptible mouse and largely inactive in other models (Noyer et al., 1995).
The high degree of stereospecificity of the anticonvulsant actions of levetiracetam suggested that the drug exerted its activity through a specific protein target. Indeed, it was soon demonstrated that [3H]levetiracetam exhibits saturable binding to a novel, highly abundant site in brain with Kd of 780 nM (Noyer et al., 1995). The affinity of levetiracetam was too low to permit the site to be further characterized. However, the azido analogue ucb 30889 was found to have 30-fold greater binding affinity, allowing it to be used as a photoaffinity fishhook. With this reagent, it was possible to identify the levetiracetam binding site as a ∼90 kDa neuron-specific protein (Fuks et al., 2003). The binding protein was widely distributed in brain, highly enriched in synaptic vesicles and likely to be integral to membranes. These characteristics raised the possibility that the binding site could be SV2A, an ubiquitous synaptic vesicle protein. This was confirmed in studies demonstrating that [3H]ucb 30889 binding is absent in mice with a targeted deletion of the SV2A gene and thus lack SV2A expression (Lynch et al., 2004). Moreover, it was shown that [3H]ucb 30889 binds to human SV2A expressed in heterologous cells and to purified SV2A. There was a high correlation between the binding affinities of a series of levetiracetam analogues and their potencies for protection against audiogenic seizures, confirming that SV2A is the molecular target for anticonvulsant activity. The functional role of SV2A is not well understood, but it seems to be a positive modulator of synaptic transmission that may act by preparing vesicles for fusion (Custer et al., 2006). It is noteworthy that SV2A knockout mice develop seizures, which leads to their demise within the first weeks of life (Crowder et al., 1999). Moreover, while heterozygous SV2A knockout mice do not show spontaneous seizures, they exhibit enhanced susceptibility to the convulsant effects of pilocarpine and kainate, a reduced 6 Hz seizure threshold and an enhanced rate of corneal kindling (Leclercq et al., 2007).
The experience with ucb 30889 indicated that substitution on the 4-position of the pyrrolidine ring of levetiracetam could enhance binding affinity. In a systematic investigation of substitutions at this site, various small hydrophobic groups were found to confer increased affinity compared with levetiracetam and enhanced activity in the audiogenic seizure test (Kenda et al., 2004). Among the analogues with enhanced binding affinity, the n-propyl analogue, brivaracetam, was selected for development as a follow-on to levetiracetam because it exhibited high antiseizure potency and favourable tolerability. [3H]Brivaracetam labels the levetiracetam binding site with Kd of 62 nM (Gillard et al., 2007), and is therefore 13-fold higher affinity than levetiracetam and 400-fold higher affinity than piracetam (Kenda et al., 2004). In this issue of the BJP, Matagne et al. (2008) now show that brivaracetam, similar to levetiracetam, has activity against kindled seizures and is more potent and possibly more efficacious. Importantly, brivaracetam confers protection in traditional suprathreshold AED screening models where levetiracetam is inactive, albeit at high doses associated with motor impairment, demonstrating that SV2A ligands as a class can have activity in these historically important and well-validated models. Levetiracetam has a remarkably wide therapeutic index of >57 in rodents, based on an ED50 for seizure protection in the audiogenic seizure-susceptible mouse of 30 mg kg−1, i.p. (Matagne et al., 2008) and a rotarod TD50 of >1700 mg kg−1, i.p., in mice and rats (Löscher and Hönack, 1993). Brivaracetam may have a reduced, but still favourable, therapeutic index of ∼23 (based on corresponding ED50 and TD50 values of 2.4 and 55 mg kg−1, respectively; Matagne et al., 2008). A recent phase II clinical trial in 208 patients demonstrated that brivaracetam (5–50 mg daily) produced a dose-dependent reduction in the frequency of seizures in adults with refractory partial seizures (French et al., 2007). Remarkably, brivaracetam had an adverse event profile indistinguishable from placebo.
In its most sophisticated form, rational drug discovery utilizes knowledge of the structure of a biomolecule involved in the pathogenesis of a disease process to engineer drugs that dock with and alter the function of the biomolecule in a desired way to ameliorate the disease (Weaver and Sankar, 2008). This paradigm has not yet been achieved in AED development. Rather, there have been some successes in optimizing the pharmacodynamic activity of prototype drugs that were identified empirically through the use of animal models. Moreover, it has become commonplace to use rational design approaches to optimize the pharmacokinetic and pharmaceutical properties of prototypes. The availability of a steady stream of molecularly diverse prototype drugs identified in animal models that are unbiased with respect to target or mechanism has, as in the case of levetiracetam, enabled the discovery of unexpected new AED targets. In addition to SV2A, promising AED targets identified in this way include α2δ proteins, which mediate the anticonvulsant and analgesic activities of gabapentin and pregabalin; KCNQ potassium channels, the target of retigabine, an AED in late stage clinical development; and collapsin response-mediating protein-2, a binding partner of lacosamide, another AED in clinical development. We have now entered a new era in AED development where screening against these and other molecular targets will play a central role in the discovery process.
Abbreviations
- AED
antiepileptic drug
References
- Crowder KM, Gunther JM, Jones TA, Hale BD, Zhang HZ, Peterson MR, et al. Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A) Proc Natl Acad Sci USA. 1999;96:15268–15273. doi: 10.1073/pnas.96.26.15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custer KL, Austin NS, Sullivan JM, Bajjalieh SM. Synaptic vesicle protein 2 enhances release probability at quiescent synapses. J Neurosci. 2006;26:1303–1313. doi: 10.1523/JNEUROSCI.2699-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JA, Brodsky A, von Rosenstiel P, on behalf of the Brivaracetam N01193 Study Group Efficacy and tolerability of 5, 20 and 50 mg/day brivaracetam (ucb 34714) as adjunctive treatment in adults with refractory partial-onset seizures Epilepsia 200749Suppl 6400(Abstract C.04)18070091 [Google Scholar]
- Fuks B, Gillard M, Michel P, Lynch B, Vertongen P, Leprince P, et al. Localization and photoaffinity labelling of the levetiracetam binding site in rat brain and certain cell lines. Eur J Pharmacol. 2003;478:11–19. doi: 10.1016/j.ejphar.2003.08.033. [DOI] [PubMed] [Google Scholar]
- Gillard M, Fuks B, Lambeng N, Chatelain P, Matagne A.Binding characteristics of brivaracetam, a novel antiepileptic drug candidate Epilepsia 200749Suppl 6317(Abstract 3.186) [Google Scholar]
- Gower AJ, Hirsch E, Boehrer A, Noyer M, Marescaux C. Effects of levetiracetam, a novel antiepileptic drug, on convulsant activity in two genetic rat models of epilepsy. Epilepsy Res. 1995;22:207–213. doi: 10.1016/0920-1211(95)00077-1. [DOI] [PubMed] [Google Scholar]
- Gower AJ, Noyer M, Verloes R, Gobert J, Wülfert E. ucb L059, a novel anti-convulsant drug: pharmacological profile in animals. Eur J Pharmacol. 1992;222:193–203. doi: 10.1016/0014-2999(92)90855-x. [DOI] [PubMed] [Google Scholar]
- Kenda BM, Matagne AC, Talaga PE, Pasau PM, Differding E, Lallemand BI, et al. Discovery of 4-substituted pyrrolidone butanamides as new agents with significant antiepileptic activity. J Med Chem. 2004;47:530–549. doi: 10.1021/jm030913e. [DOI] [PubMed] [Google Scholar]
- Klitgaard H, Matagne A, Gobert J, Wülfert E. Evidence for a unique profile of levetiracetam in rodent models of seizures and epilepsy. Eur J Pharmacol. 1998;353:191–206. doi: 10.1016/s0014-2999(98)00410-5. [DOI] [PubMed] [Google Scholar]
- Kohn A. Fortune or Failure: Missed Opportunities and Chance Discoveries. Basil Blackwell: Oxford, UK; 1989. [Google Scholar]
- Leclercq K, Kaminski R, Dassesse D, Klitgaard H, Matagne A.Seizure susceptibility of SV2A heterozygous mice in models of temporal lobe epilepsy 2007Society for Neuroscience: San Diego, CA; Program No. 492.17. 2007 Neuroscience Meeting Planner.Online [Google Scholar]
- Löscher W, Hönack D. Profile of ucb L059, a novel anticonvulsant drug, in models of partial and generalized epilepsy in mice and rats. Eur J Pharmacol. 1993;232:147–158. doi: 10.1016/0014-2999(93)90768-d. [DOI] [PubMed] [Google Scholar]
- Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjalieh SM, Matagne A, et al. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci USA. 2004;101:9861–9866. doi: 10.1073/pnas.0308208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matagne A, Margineanu D, Kenda B, Michel P, Klitgaard H.Anti-convulsive and anti-epileptic properties of brivaracetam (ucb 34714), a high-affinity ligand for the synaptic vesicle protein SV2A Br J Pharmacol 20081541662–1671.this issue [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyer M, Gillard M, Matagne A, Hénichart JP, Wülfert E. The novel antiepileptic drug levetiracetam (ucb L059) appears to act via a specific binding site in CNS membranes. Eur J Pharmacol. 1995;286:137–146. doi: 10.1016/0014-2999(95)00436-o. [DOI] [PubMed] [Google Scholar]
- Weaver DF, Sankar R.Basic principles of medicinal chemistry Epilepsy: A Comprehensive Textbook 2008Lippincott Williams & Wilkins: Philadelphia; 1447–1455.In: Engel Jr J, Pedley TA (eds).2nd edn. [Google Scholar]