Figure 1.
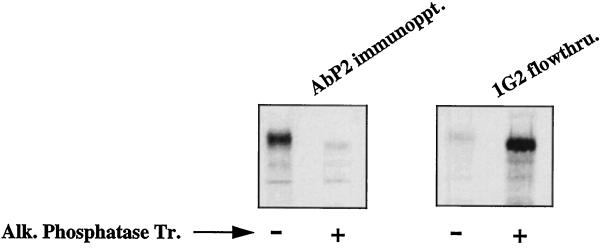
The phosphorylation-induced conformational change is reversible. A431 cells were labeled with Tran 35S-label for 10 h. The EGF receptors in the detergent-solubilized cell lysates were phosphorylated with unlabeled ATP in the presence of 1 μM EGF as described in MATERIALS AND METHODS. After purification of the EGF receptors by anti-phosphotyrosine monoclonal antibody (1G2), the receptors were allowed to bind with wheat germ agglutinin–agarose. The bound receptor was eluted from the beads by 0.4 M N-acetylglucosamine in 20 mM HEPES, pH 7.4, 0.15 M NaCl, 0.2% NP-40, and protease inhibitors. An aliquot of the purified receptor was left untreated (−) or treated with alkaline phosphatase (50 units/ml) (+) coupled to agarose in the presence of 1 mg/ml bovine serum albumin. After incubation at 4°C for 1 h, both samples were centrifuged, and the supernatants were subjected to immunoprecipitation in the presence of 1 mM vanadate with Ab P2 (AbP2 immunoppt.; left) or anti-phosphotyrosine antibody 1G2 (right). 1G2 flowthru. denotes the labeled receptor that did not bind to 1G2. Alk. Phosphatase Tr., Alkaline phosphatase treatment.