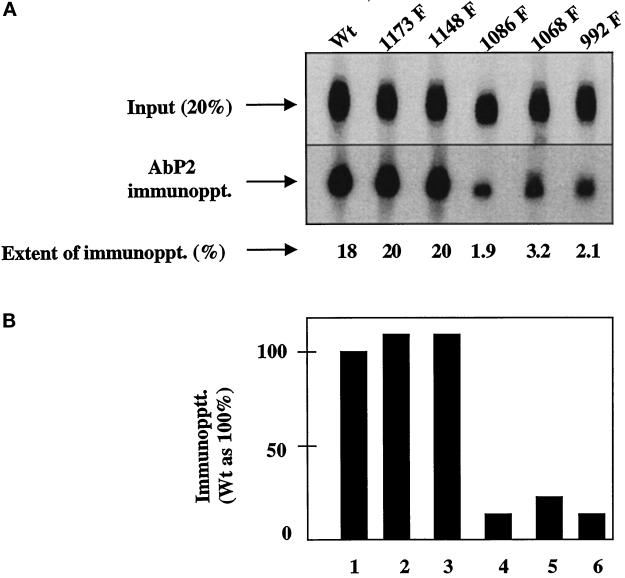
Figure 3.
Single Tyr→Phe substitution at 992, 1068, or 1086 drastically reduces the binding of Ab P2 to the 32P-labeled EGF receptor. Detergent-solubilized membranes from the wild type (Wt) or the single Y→F EGF receptor mutants were phosphorylated with labeled ATP (specific radioactivity of 350 cpm/fmol) in the presence of 1 μM EGF. After purification of the labeled receptor by 1G2–Sepharose, the 32P-labeled receptor was quantified as described in MATERIALS AND METHODS. For immunoprecipitation, 1.25 fmol of the EGF receptor was incubated with 5 μg of protein A–purified Ab P2 in a total volume of 15 μl under conditions described in MATERIALS AND METHODS. After isolation of the immune complexes with formaldehyde-fixed S. aureus, the labeled proteins were analyzed by SDS-PAGE and autoradiography, and the region containing the 170-kDa EGF receptor band was densitometrically scanned. (A) Top, 20% of labeled samples that have not been subjected to immunoprecipitation [Input (20%)]. Middle, the EGF receptor bands after immunoprecipitation with Ab P2 (AbP2 immunoppt.). Bottom, the extent of immunoprecipitation. (B) The results of the immunoprecipitation relative to that of the wild-type receptor.