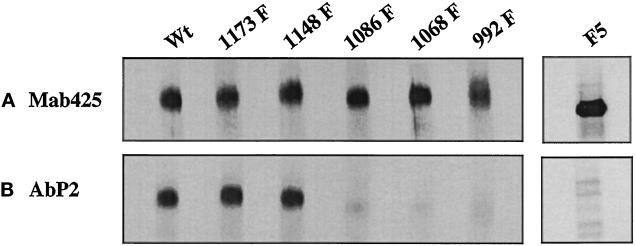
Figure 4.
Interaction of Ab P2 with 35S-labeled EGF receptor is dependent on the phosphorylation of Tyr 992, 1068, and 1086. 35S-labeled EGF receptors from the wild type and the single Y→F substitution mutants were phosphorylated with unlabeled ATP in the presence of EGF, and the labeled receptors were purified with 1G2–Sepharose. (A) The relative concentrations of the EGF receptor in different cell types were quantified by precipitation with mAb 425 (Mab425), a monoclonal antibody to an external peptide epitope of the receptor. (B) Equal amounts of the EGF receptor from different mutants as shown in A were subjected to immunoprecipitation with Ab P2 (AbP2) followed by SDS-PAGE (3.5–10%) and fluorography. In a separate experiment, 35S-labeled cell lysates from the EGF receptor F5 mutant in which all five known phosphate acceptor sites were mutated to Phe were incubated with unlabeled ATP in the presence of EGF, and the labeled receptors were purified with wheat germ agglutinin. The purified receptor preparation was immunoprecipitated with mAb 425, Ab P2, or nonimmune serum and was analyzed by SDS-PAGE as described above.