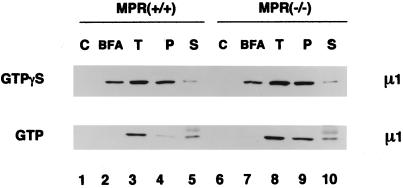
Figure 6.
Sensitivity of bound AP-1 to extraction with Tris-HCl. AP-1 recruitment assays containing cytosol, normal (+/+) or MPR-negative (−/−) Golgi-enriched membranes, and exogenous ARF1 were performed in the absence of nucleotides, or in the presence of either 100 μM GTPγS or 1 mM GTP, with or without 100 μg/ml BFA. After incubation, pellets from assays without added nucleotides (C), or with guanine nucleotide and BFA together (BFA), or with guanine nucleotide alone (T) were recovered and resuspended in SDS-sample buffer. A fourth pellet, identical to that in sample T, was resuspended in 100 μl of 1.0 M Tris-HCl, pH 7.0, on ice. After incubation on ice for 10 min, the membranes were collected again by centrifugation. The resulting supernatant was aspirated and protein was precipitated with methanol/chloroform after addition of 5 μg BSA as a carrier. The pellet (P) and supernatant (S) samples from the Tris extraction were resuspended in identical volumes of SDS-sample buffer and, together with the other pellets, analyzed by immunoblotting with the anti–AP-1 μ1-subunit antibody, RY/1. The diffuse band above the μ1 subunit seen in the lower panel (lanes 5 and 10) corresponds to nonspecific reactivity with the carrier BSA.