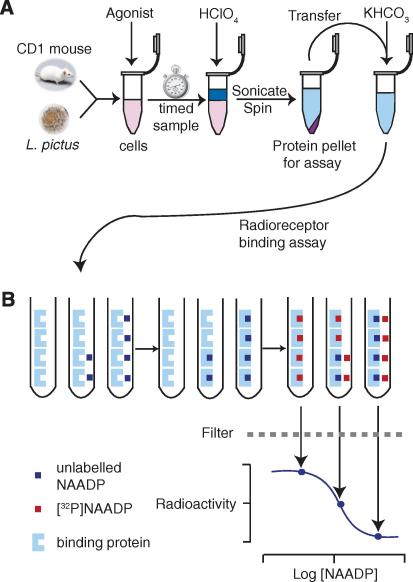
Fig. 1.
(A) Schematic diagram of the acid extraction process for NAADP. Sperm or eggs are harvested from L. pictus, or cells are prepared from male CD1 mouse pancreas. Agonist is incubated with the cell preparation, and then HClO4 is added to stop the reaction at the required time point. The sample is then sonicated to disrupt the cells and is centrifuged to pellet the protein for subsequent assay. The supernatant is neutralized with an equal volume of 2 M KHCO3, or other bases where indicated, in preparation for analysis using the radioreceptor binding assay. (B) Schematic diagram of the NAADP radioreceptor binding assay. First, known concentrations of NAADP (blue boxes), or cell extracts, are added, followed by sea urchin egg homogenate (pale blue shapes) in intracellular medium and a 10-min incubation period. NAADP binds irreversibly to the receptors in the homogenate. [32P]NAADP (red boxes) is then added. This binds to the remaining available receptor sites. Bound NAADP is separated from the mixture by filtration, and the radioactivity is determined. Sample NAADP concentrations may be determined from the standard curve. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)