Figure 1.
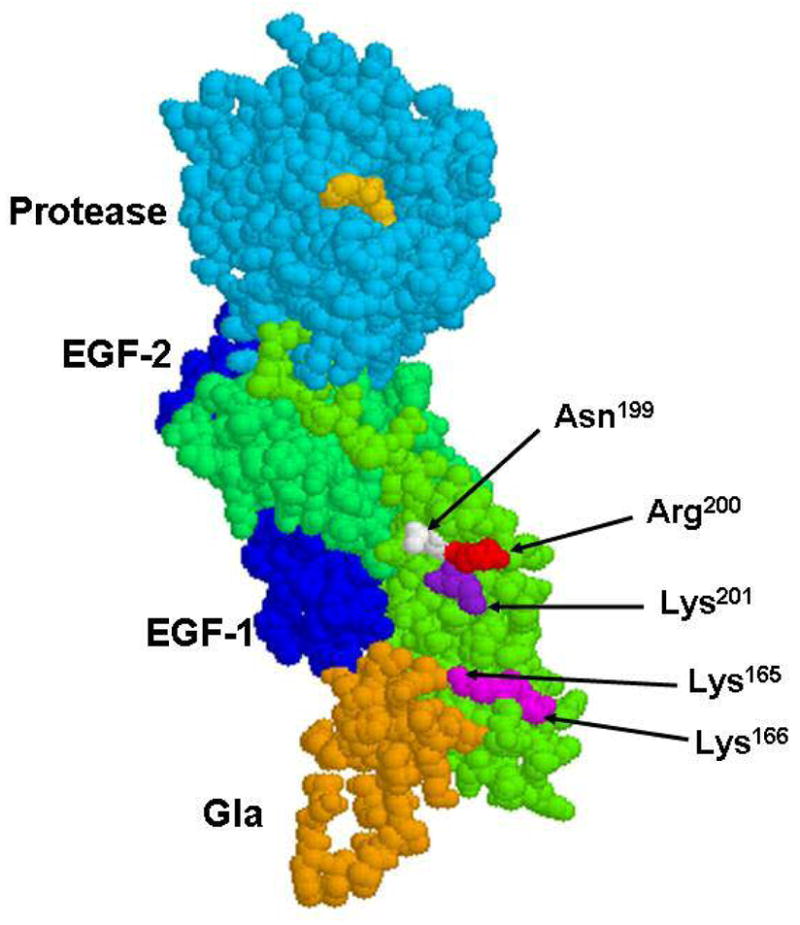
The space-filling model of the crystal structure of the fVIIa-sTF complex. The side chains of three residues Asn-199, Arg-200 and Lys-201 are shown by arrows. The side chains of Lys-165 and Lys-166 which are thought to interact with the Gla-domain of fVIIa (19,20) are also shown. The sTF residues are colored in green, the residues of the Gla-domain of fVIIa are colored in gold, EGF-1 and EGF-2 are in dark blue and the protease domain of the PPACK-inhibited fVIIa is shown in cyan. The inhibitor in the active-site of fVIIa is shown in yellow. The coordinates (Protein Data Bank accession code 1DAN) were used to prepare the figure (12).
