Abstract
Mutational loss of pesticin I, a bacteriocin-like substance produced by Pasteurella pestis, is known to result in concomitant loss of a coagulase and fibrinolytic factor. No relationship was detected between pesticinogeny and other tested properties either associated with virulence or peculiar to P. pestis. Pesticin I was distinguished from the coagulase and fibrinolytic activities on the basis of anatomical distribution, behavior during gel filtration, and sensitivity to heat. Coagulase and the fibrinolytic factor were not differentiated by these criteria. Spontaneous suppressor mutations causing reversion to pesticinogeny were not detected, nor were such mutants obtained by treatment with ultraviolet light or 2-aminopurine. Attempts to demonstrate a common activator of pesticin I, coagulase, or the fibrinolytic factor in extracts of pesticinogenic cells were not successful. These results are in accord with the hypothesis that at least two structural genes for the three activities reside on a replicon distinct from the chromosome proper. Fibrinolytic activity was significantly reduced in the presence of 0.003 m ε-aminocaproic acid and was nonexistent on fibrin films freed from endogenous plasminogen by treatment with heat. Fibrinolytic activity on heated films could be restored by addition of plasma or serum from six mammalian species. Accordingly, the plague fibrinolytic factor, like staphylokinase or urokinase, promotes the conversion of plasminogen to plasmin.
Full text
PDF


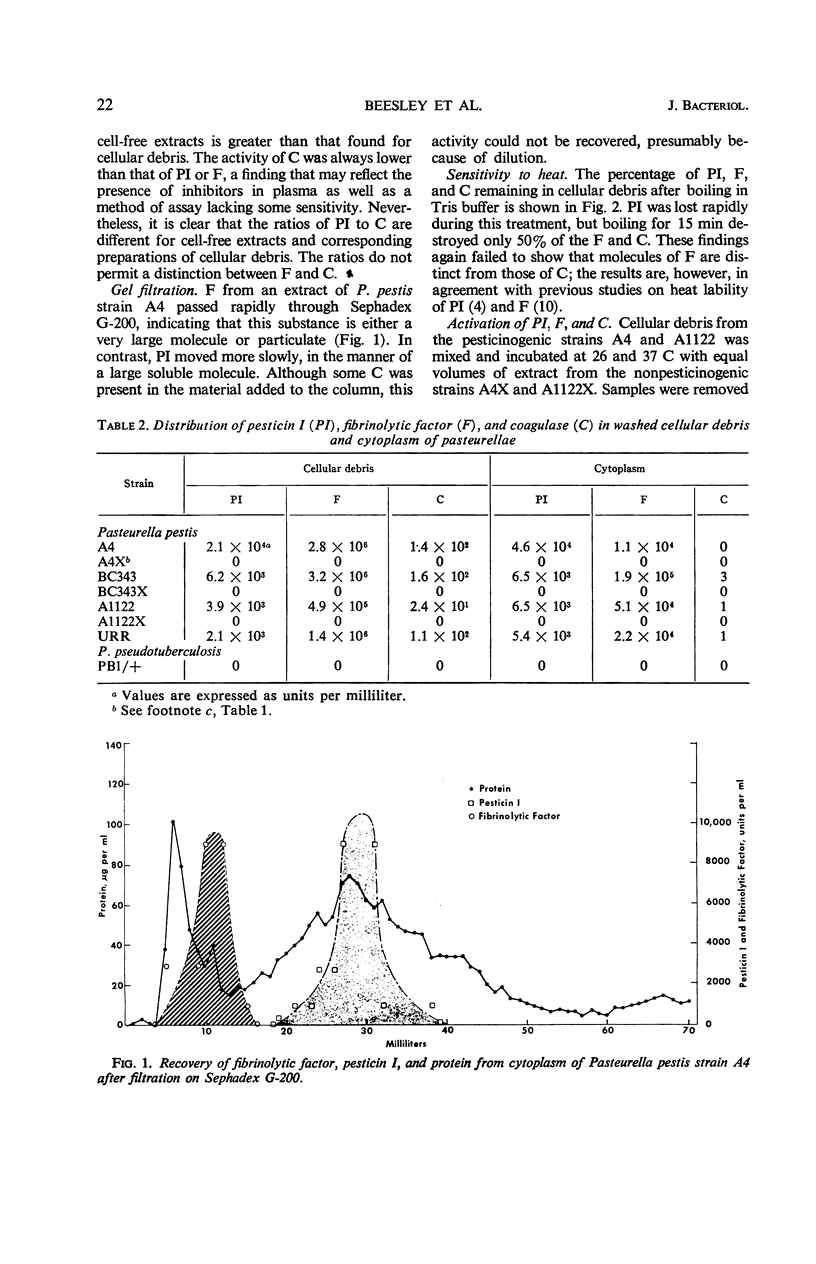




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALKJAERSIG N., FLETCHER A. P., SHERRY S. The activation of human plasminogen. II. A kinetic study of activation with trypsin, urokinase, and streptokinase. J Biol Chem. 1958 Jul;233(1):86–90. [PubMed] [Google Scholar]
- ALKJAERSIG N., FLETCHER A. P., SHERRY S. xi-Aminocaproic acid: an inhibitor of plasminogen activation. J Biol Chem. 1959 Apr;234(4):832–837. [PubMed] [Google Scholar]
- ASTRUP T., MULLERTZ S. The fibrin plate method for estimating fibrinolytic activity. Arch Biochem Biophys. 1952 Oct;40(2):346–351. doi: 10.1016/0003-9861(52)90121-5. [DOI] [PubMed] [Google Scholar]
- BEN-GURION R., HERTMAN I. Bacteriocin-like material produced by Pasteurella pestis. J Gen Microbiol. 1958 Oct;19(2):289–297. doi: 10.1099/00221287-19-2-289. [DOI] [PubMed] [Google Scholar]
- BRUBAKER R. R., SURGALLA M. J. Pesticins. I. Pesticinbacterium interrelationships, and environmental factors influencing activity. J Bacteriol. 1961 Dec;82:940–949. doi: 10.1128/jb.82.6.940-949.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUBAKER R. R., SURGALLA M. J. Pesticins. II. Production of pesticin I and II. J Bacteriol. 1962 Sep;84:539–545. doi: 10.1128/jb.84.3.539-545.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURROWS T. W., JACKSON S. The pigmentation of Pasteurella pestis on a defined medium containing haemin. Br J Exp Pathol. 1956 Dec;37(6):570–576. [PMC free article] [PubMed] [Google Scholar]
- Brubaker R. R., Beesley E. D., Surgalla M. J. Pasteurella pestis: Role of Pesticin I and Iron in Experimental Plague. Science. 1965 Jul 23;149(3682):422–424. doi: 10.1126/science.149.3682.422. [DOI] [PubMed] [Google Scholar]
- DOMARADSKII I. V., IAROMIUK G. A., VASIUKHINA L. V., KOROTAEVA A. V. O KOAGULIATSII PLAZMY KROVI CHUMNYM I PSEVDOTUBERKULEZNYM MIKROBAMI. Biull Eksp Biol Med. 1963 Jul;56:79–82. [PubMed] [Google Scholar]
- HERTMAN I., BEN-GURION R. A study on pesticin biosynthesis. J Gen Microbiol. 1959 Aug;21:135–143. doi: 10.1099/00221287-21-1-135. [DOI] [PubMed] [Google Scholar]
- HIGUCHI K., SMITH J. L. Studies on the nutrition and physiology of Pasteurella pestis. VI. A differential plating medium for the estimation of the mutation rate to avirulence. J Bacteriol. 1961 Apr;81:605–608. doi: 10.1128/jb.81.4.605-608.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- KAPRAL F. A., LI I. W. Virulence and coagulases of Staphylococcus aureus. Proc Soc Exp Biol Med. 1960 May;104:151–153. doi: 10.3181/00379727-104-25761. [DOI] [PubMed] [Google Scholar]
- KNAPP W. KLINISCH-BAKTERIOLOGISCHE UND EPIDEMIOLOGISCHE BEFUNDE BEI DER PSEUDOTUBERKULOSE DES MENSCHEN. Arch Hyg Bakteriol. 1963 Aug;147:369–380. [PubMed] [Google Scholar]
- LASSEN M. Heat denaturation of plasminogen in the fibrin plate method. Acta Physiol Scand. 1953 Feb 28;27(4):371–376. doi: 10.1111/j.1748-1716.1953.tb00951.x. [DOI] [PubMed] [Google Scholar]
- LAWTON W. D., FUKUI G. M., SURGALLA M. J. Studies on the antigens of Pasteurella pestis and Pasteurella pseudotuberculosis. J Immunol. 1960 May;84:475–479. [PubMed] [Google Scholar]
- LEWIS J. H., FERGUSON J. H. Studies on a proteolytic enzyme system of the blood. I. Inhibition of fibrinolysin. J Clin Invest. 1950 Apr;29(4):486–490. doi: 10.1172/JCI102284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MASSHOFF W. [Pseudotuberculosis in man]. Dtsch Med Wochenschr. 1962 May 4;87:915–920. doi: 10.1055/s-0028-1111847. [DOI] [PubMed] [Google Scholar]
- MULLERTZ S., LASSEN M. An activator system in blood indispensable for formation of plasmin by streptokinase. Proc Soc Exp Biol Med. 1953 Feb;82(2):264–268. doi: 10.3181/00379727-82-20087. [DOI] [PubMed] [Google Scholar]
- PATERSON J. S., COOK R. A method for the recovery of Pateurella pseudotuberculosis from faeces. J Pathol Bacteriol. 1963 Jan;85:241–242. doi: 10.1002/path.1700850124. [DOI] [PubMed] [Google Scholar]
- REEVES P. THE BACTERIOCINS. Bacteriol Rev. 1965 Mar;29:24–45. doi: 10.1128/br.29.1.24-45.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed G. B., Orr J. H., Brown H. J. Fibrinolysins from Gas Gangrene Anaerobes. J Bacteriol. 1943 Nov;46(5):475–480. doi: 10.1128/jb.46.5.475-480.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHERRY S., FLETCHER A. P., ALKJAERSIG N. Fibrinolysis and fibrinolytic activity in man. Physiol Rev. 1959 Apr;39(2):343–382. doi: 10.1152/physrev.1959.39.2.343. [DOI] [PubMed] [Google Scholar]
- SMITH D. A., BURROWS T. W. Phage and bacteriocin investigations with Pasteurella pestis and other bacteria. Nature. 1962 Jan 27;193:397–398. doi: 10.1038/193397a0. [DOI] [PubMed] [Google Scholar]



