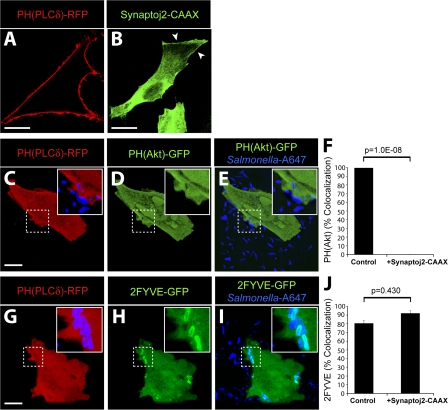
Figure 4.
PI(3)P formation on the SCV is not dependent on PI(3,4)P2 and PI(3,4,5)P3 production at invasion ruffles. (A and B) Localization of PH(PLCδ)-RFP (A) and synaptojanin-2–CAAX construct (B) in control cells. Arrowheads indicate plasma membrane localization of synaptojanin-2–CAAX construct. (C–F) Cells were cotransfected with synaptojanin-2–CAAX, PH(PLCδ)-RFP (C), and PH(Akt)-GFP (D). (E) Merged image showing localization of bacteria (labeled with Alexa Fluor 647) relative to signal for PH(Akt)-GFP. (F) Cells were cotransfected as in C–E, infected with WT S. typhimurium, and analyzed by confocal microscopy. Images were acquired at 1-min intervals for ∼1 h. Colocalization of the bacteria with PH(Akt)-GFP during infection is shown. As a control, cells were cotransfected with PH(PLCδ)-RFP and PH(Akt)-GFP but not synaptojanin-2–CAAX. Data are means ± SEM of three separate experiments for synaptojanin-2–CAAX-expressing cells (20 ruffles analyzed) and two separate experiments for control cells (13 ruffles analyzed). The p-value is shown. (G–J) Cells were cotransfected with synaptojanin-2–CAAX, PH(PLCδ)-RFP (G), and 2FYVE-GFP (H). (I) Merged image showing localization of bacteria (labeled with Alexa Fluor 647) relative to signal for 2FYVE-GFP. Insets are enlarged from dashed boxes. (J) Cells were cotransfected as in G–I, infected with WT bacteria, and analyzed by confocal microscopy. Images were acquired at 1-min intervals for at least 1 h. Colocalization of the bacteria with 2FYVE-GFP during infection is shown. As a control, cells were cotransfected with PH(PLCδ)-RFP and 2FYVE-GFP but not synaptojanin-2–CAAX. Data are means ± SEM of three separate experiments for synaptojanin-2–CAAX- expressing cells (111 SCVs analyzed) and two separate experiments for control cells (44 SCVs analyzed). The p-value is shown. Bars, 10 μm.