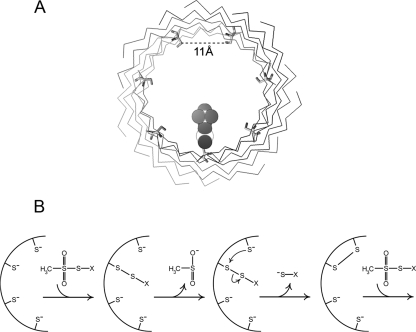
Figure 9.
(A) View through the β-barrel of α-hemolysin, with the cis end proximal. Cys was substituted for Asp at residue 127 using the “mutagenesis” feature of PyMol (with no subsequent energy minimization); this roughly corresponds to residue S312 in the PA sequence. The 11 Å distance displayed is between β carbons from adjacent subunits and represents an average of the seven nearest neighbor distances in the crystal structure of α-hemolysin. A sulfur-ethyltrimethylammonium adduct was drawn using ChemDraw, scaled appropriately, and placed in the pore at one of those residues to mimic the mixed disulfide generated by reaction of this cys residue with MTSET. (B) Proposed scheme for MTS-catalyzed intramolecular disulfide bond formation in cysteine-substituted channels. View is of a cross section of the barrel, with only a portion shown. A single MTS-X reagent reacts with one of the seven free thiols in the (PA63)7 pore to generate the mixed disulfide PA-S-S-X. This disulfide can then participate in thiol exchange with a neighboring cysteine to generate an intersubunit disulfide, releasing the S-X group in the process. Another MTS-X molecule could potentially enter the pore to react with one of the five remaining thiols to catalyze additional disulfide reactions.