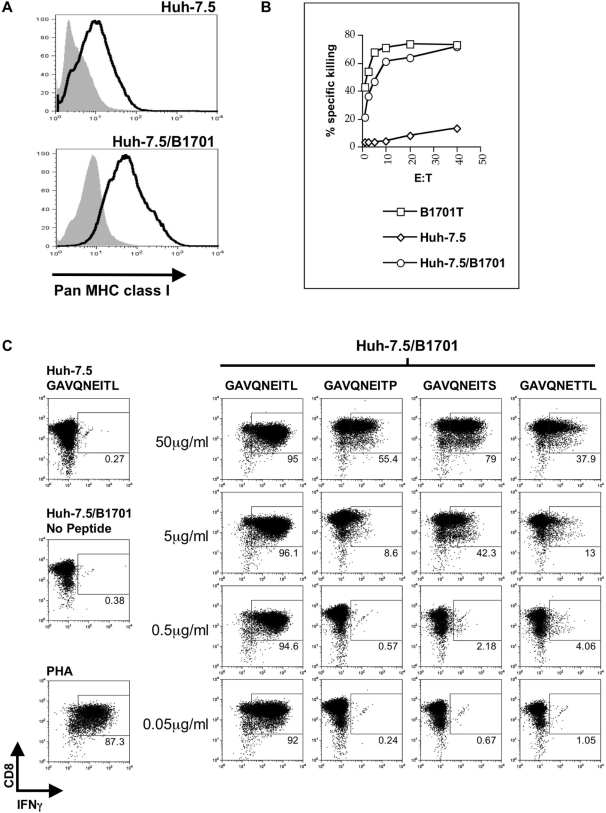
Figure 2. Expression and recognition of the chimpanzee Patr-B1701 molecule on the surface of Huh-7.5 cells.
(A) Surface expression of MHC class I on Huh-7.5 cells transfected with a plasmid containing the Patr-B1701 molecule and zeocin selection marker. No difference in surface expression was observed when compared to untransfected Huh-7.5 cells. Isotype control is depicted in grey. (B) CTL lysis of transfected Huh-7.5 cells. Huh-7.5 cells expressing the Patr-B1701 (Huh-7.5/B1701) molecule were pulsed with wild-type peptide and incubated with increasing amounts of CTL clone 4A specific for the NS31629–1637 wild-type epitope. Cells presenting this peptide on the Patr-B1701 molecule are lysed by CTLs as efficiently as EBV-transformed autologous B cells presenting peptide (B1701T). Untransfected Huh-7.5 cells served as a negative control. (C) CD8+ T cell clone IFNγ response to Huh-7.5/B1701 cells presenting exogenous peptide. Huh-7.5/B1701 cells were loaded with parent HCV1/910 NS31629–1637 or mutant NS31629–1637 peptide as in (B) and cocultured with a CD8+ T cell clone targeting the NS31629–1637 epitope. Huh-7.5/B1701 cells presenting parent HCV1/910 NS31629–1637 but not mutant peptide at concentrations of 0.5 µg/ml and lower could elicit an IFNγ response from the CD8+ T clone. Cocultures were stimulated with PHA as a positive control, and unpulsed Huh-7.5/B1701 cells or Huh-7.5 cells pulsed with parent HCV1/910 NS31629–1637 peptide served as negative controls. Plots depicted are gated on CD3+ T cells.