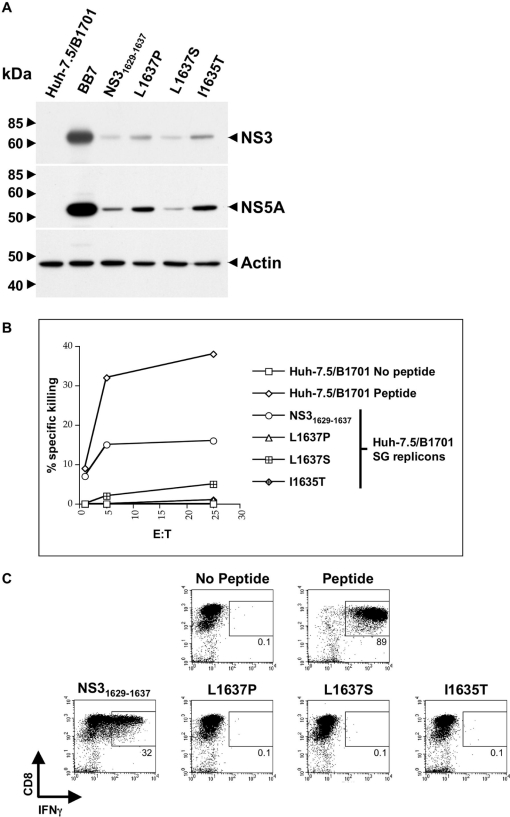
Figure 3. Mutations in the NS31629–1637 epitope abrogate CTL recognition in the replicon system.
(A) Western blots of replicon-transfected Patr-B1701-expressing Huh-7.5 cells. The expression of HCV proteins NS3 and NS5A were detected in Huh-7.5/B1701 cells harboring subgenomic replicons. Lysates from Huh-7.5/B1701 cells transfected with replicons containing the BB7 epitope served as a positive control, untransfected Huh-7.5/B1701 cells were used as a negative control, and β-actin served as a positive control for input protein. (B) CTLs are unable to lyse Huh-7.5/B1701 cells transfected with subgenomic mutant replicons with the same efficiency as the parent HCV1/910 NS31629–1637 subgenomic replicon. Huh-7.5/B1701 cells with or without parent HCV1/910 NS31629–1637 peptide were used as a positive and negative control, respectively. (C) CD8+ T cell IFNγ response to the mutated NS31629–1637 epitope in the subgenomic system. CD8+ T cells specific for the wild-type NS31629–1637 epitope were incubated with Huh-7.5/B1701 cells transfected with either the parent HCV1/910 NS31629–1637 subgenomic replicon or the mutant subgenomic replicons, stained for CD8 and IFNγ, and analyzed by flow cytometry. CD8+ T cells secrete IFNγ in response to the parent HCV1/910 NS31629–1637 subgenomic-transfected Huh-7.5/B1701 cells, but are unable to secrete IFNγ when incubated with Huh-7.5/B1701 cells harboring the subgenomic mutant replicons. Huh-7.5/B1701 cells were incubated with or without the parent HCV1/910 NS31629–1637 peptide as a positive and negative control, respectively.