Abstract
Previously, we found that high intraluminal pressure leads to production of reactive oxygen species (ROS) and also upregulates several components of the renin-angiotensin system in the wall of small arteries. We hypothesized that acute exposure of arterioles to high intraluminal pressure in vitro via increasing ROS production enhances the functional availability of type 1 angiotensin II (Ang II) receptors (AT1 receptors), resulting in sustained constrictions. In arterioles (∼180 μm) isolated from rat skeletal muscle, Ang II elicited dose-dependent constrictions, which decreased significantly by the second application [maximum (max.): from 59% ± 4% to 26% ± 5% at 10−8 M; P < 0.05] in the presence of 80 mmHg of intraluminal pressure. In contrast, if the arterioles were exposed to high intraluminal pressure (160 mmHg for 30 min), Ang II-induced constrictions remained substantial on the second application (max.: 51% ± 3% at 10−8 M). In the presence of Tiron and polyethylene glycol (PEG)-catalase, known to reduce the level of superoxide anion and hydrogen peroxide (H2O2), second applications of Ang II evoked similarly reduced constrictions, even after high-pressure exposure (29% ± 4% at 10−8 M). Furthermore, when arterioles were exposed to H2O2 (for 30 min, 10−7 M, at normal 80 mmHg pressure), Ang II-induced constrictions remained substantial on second applications (59% ± 5% at 10−8 M). These findings suggest that high pressure, likely via inducing H2O2 production, increases the functional availability of AT1 receptors and thus enhances Ang II-induced arteriolar constrictions. We propose that in hypertension–regardless of etiology–high intraluminal pressure, via oxidative stress, enhances the functional availability of AT1 receptors augmenting Ang II-induced constrictions.
Keywords: hypertension, oxidative stress, hydrogen peroxide
there are several forms of hypertension, in which the activity of the systemic renin angiotensin system (RAS) or circulating levels of angiotensin II (Ang II) are not primarily responsible for the elevated peripheral vascular resistance (5, 22). Yet many clinical trials have shown that angiotensin-converting enzyme (ACE) inhibitors or Ang II type 1 (AT1) receptor blockers efficiently reduce blood pressure and prevent vascular complications almost in all forms of hypertension (3, 4, 29, 31).
Early studies have shown a complex interaction between intraluminal pressure and local vasoregulatory function in the arteriolar wall (9, 19, 20). We (27) have found recently that in aortic banded rats, high pressure upregulates tissue ACE in arteries that are exposed to high intraluminal pressure but not in those exposed to normal pressure of the same rats, even though both types of vessels are exposed to the same circulating factors. A key role for the increased high pressure-induced production of reactive oxygen species (ROS) has been implicated in this process (27) as an underlying mechanism known to play a crucial role in hypertension-related vascular pathology (10, 11, 14, 15). Moreover, these findings suggested a crucial role for pressure-sensitive upregulation of RAS in the wall of resistance vessels, independent of other confounding circulating factors, which can also be present in hypertension. Thus it is plausible that high pressure itself interacts with the elements of vascular RAS leading to an enhanced, local production of Ang II to exaggerate its well-known detrimental vascular effects (18), independent of systemic RAS.
It is known that Ang II via activating AT1 receptors elicits pronounced constriction in resistance vessels (18) but is, however, markedly diminished on repeated application, known as Ang II tachyphylaxis (13, 17, 24, 28). A unique feature of AT1 receptors is that they undergo rapid desensitization and consequent internalization on stimulation by Ang II (12, 16). Normally, this mechanism limits the availability of the active AT1 receptors on the cell surface and consequently the regulation of vascular resistance (7). It is possible that in various pathological conditions, such as in hypertension, this normal regulation of the AT1 receptor function is altered, which may lead to a sustained availability of active AT1 receptors. Yet the functional evidence supporting this idea and the possible underlying mechanisms are still lacking. Thus, in the present study, we have tested the hypothesis that short-term exposure of resistance arteries to high intraluminal pressure via eliciting enhanced production of ROS leads to sustained constrictions to Ang II via increased availability of AT1 receptors.
METHODS
Isolation of skeletal muscle arteries.
All protocols were approved by the Institutional Animal Care and Use Committee at New York Medical College, Valhalla. Male Wistar rats (weighting ∼300 g, n = 40) were anesthetized with an intraperitoneal injection of pentobarbital sodium (50 mg/kg). Under anesthesia, gracilis muscles were excised and placed in ice-cold, oxygenated Krebs solution. Euthanasia was then performed by additional intraperitoneal injection of pentobarbital sodium (150 mg/kg). With the use of microsurgical instruments and an operating microscope, the gracilis arterioles (∼1.5 mm in length) of rats were isolated and cannulated as described previously (10, 11, 14, 15). The cannulated arteries were connected with silicone tubing to a pressure servo control system (Living Systems Instrumentation) to adjust the intraluminal pressure. Vessel diameter was continuously recorded with a digital camera (CFW1310, Scion) connected to a microscope (Eclipse 80i, Nikon). Images were stored and analyzed with a computer.
Experimental protocols.
In the first series of experiments, in the presence of 80 mmHg, cumulative concentrations of Ang II (1 nmol/l to 0.1 μmol/l; Sigma) and norepinephrine (NE; 1 nmol/l to 0.1 μmol/l; Sigma) were administered to the vessels, and changes in diameter were measured. Thirty minutes after washout, agonist-induced responses were repeated.
In separate protocols, repeated agonist-induced responses were also obtained in vessels, which were transiently exposed to high intraluminal pressure (160 mmHg for 30 min) before the second drug application. In similar protocols, vasoconstrictions to Ang II and NE were assessed in the presence of the selective Ang II type 2 (AT2) receptor inhibitor, PD-123319 (10 μmol/l for 30 min). Responses to Ang II were assessed in the presence of the selective AT1 receptor blocker, telmisartan (10 μmol/l for 30 min).
In other sets of experiments, repeated agonist-induced vasomotor responses were obtained in the presence of Tiron and polyethylene glycol (PEG)-catalase (10 μmol/l and 200 U/ml for 30 min, respectively), both in normal and high pressure-exposed arterioles, to reveal the contribution of ROS in the altered agonist-induced arteriolar responsiveness. In similar protocols, arterioles were transiently exposed to hydrogen peroxide (H2O2; 0.1 μmol/l for 30 min), and agonist-induced arteriolar responses were obtained again in the presence of 80 mmHg pressure.
Data analysis.
Data are expressed as means ± SE. Agonist-induced vasoconstrictions were expressed as percent changes in arterial diameter. Statistical analyses were performed using GraphPad Prism Software (San Diego, CA) by repeated measures ANOVA followed by Tukey's post hoc test. P < 0.05 was considered statistically significant.
RESULTS
Intraluminal pressure and arterial constrictions to repeated applications of Ang II.
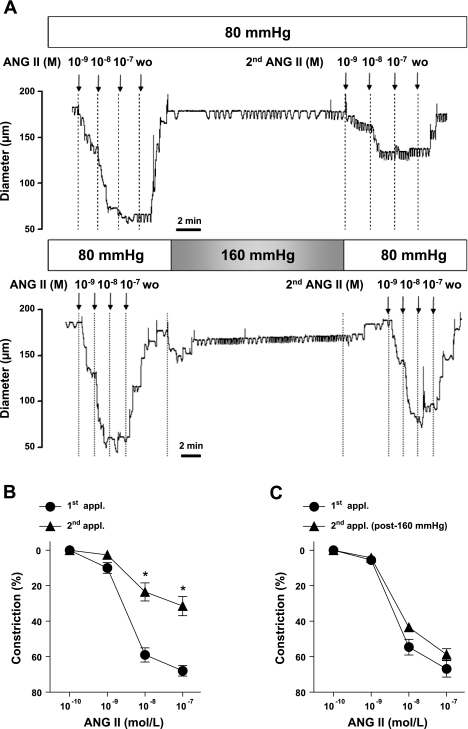
Original records show that in the presence of 80 mmHg intraluminal pressure increasing concentrations of Ang II elicited substantial constrictions in isolated skeletal muscle arterioles. These constrictions were primarily mediated by AT1 receptor activation since presence of the AT1 receptor blocker, telmisartan, markedly reduced constrictions to Ang II [maximum (max.) constrictions: before telmisartan, 64% ± 5%; after telmisartan, 3% ± 4%]. After 30 min, in response to the second application, Ang II elicited greatly diminished vasoconstrictions (Fig. 1A) in the continuous presence of 80 mmHg intraluminal pressure. If, however, the vessel was exposed transiently to high intraluminal pressure (160 mmHg for 30 min and then returned to 80 mmHg), the magnitude of constrictions to the second application of Ang II remained close to control (Fig. 1A). Summary data show that compared with the responses of vessels exposed to normotensive pressure, exposure of vessels to high intraluminal pressure significantly augmented the constrictions to the second application of Ang II (Fig. 1, B and C).
Fig. 1.
Original records (A) and summary data (n = 11; B) of constrictions of arterioles to repeated applications (appl.) of angiotensin II (Ang II; 0.1 nmol/l to 0.1 μmol/l) in the presence of 80 mmHg and after exposure of 160 mmHg intraluminal pressure (n = 10; C). WO, washout. Data are means ± SE. *P < 0.05.
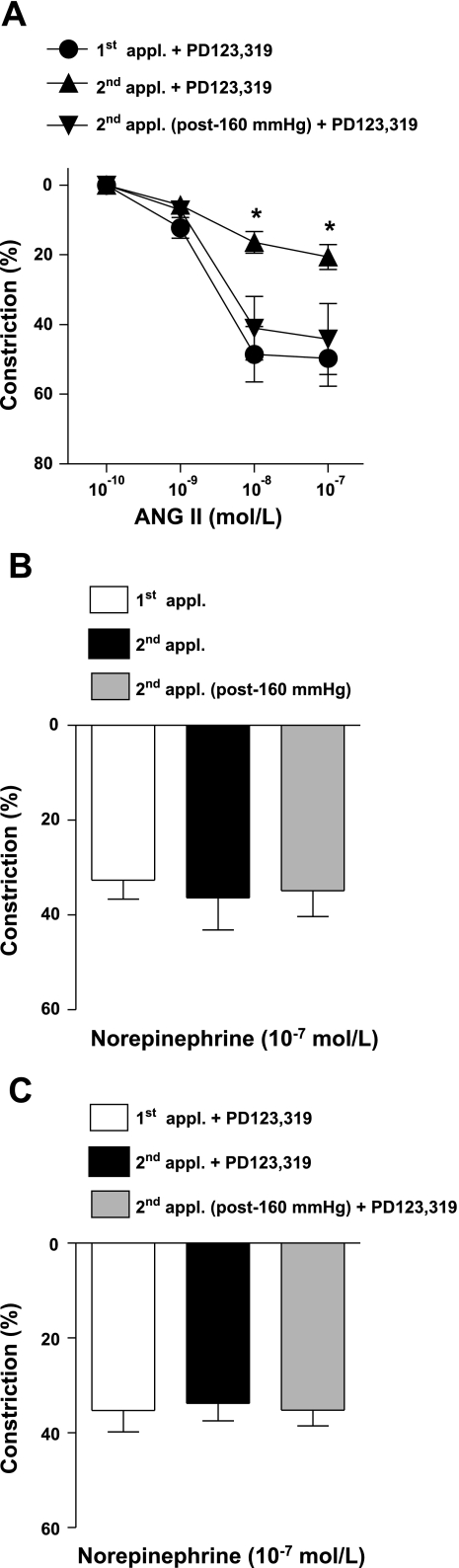
In similar conditions, arteriolar constrictions to Ang II were also measured in the presence of the selective AT2 receptor inhibitor, PD-123319, to reveal possible, if any, involvement of high pressure-dependent activation of AT2 receptors. Presence of PD-123319, however, did not significantly affect Ang II-induced constrictions to exposure of either 80 or 160 mmHg pressure (Fig. 2A). Moreover, we have found that constrictions to the repeated administration of NE were maintained in the presence of normal 80 mmHg pressure and were not affected if arterioles were exposed to high intraluminal pressure or by the presence of PO-123319 (Fig. 2, B and C).
Fig. 2.
Constrictions of arterioles to repeated applications of Ang II (0.1 nmol/l to 0.1 μmol/l) in the presence of the Ang II type 2 (AT2) receptor antagonist, PD-123319, at 80 mmHg and after exposure of 160 mmHg pressure (n = 7; A). Constrictions of arterioles to repeated applications of norepinephrine (0.1 μmol/l) in the absence (n = 7; B) and presence (n = 7; C) of the AT2 receptor antagonist, PD-123319, at 80 mmHg and after exposure of 160 mmHg pressure. Data are means ± SE. *P < 0.05.
Role of ROS in mediating pressure-induced sustained Ang II responsiveness.
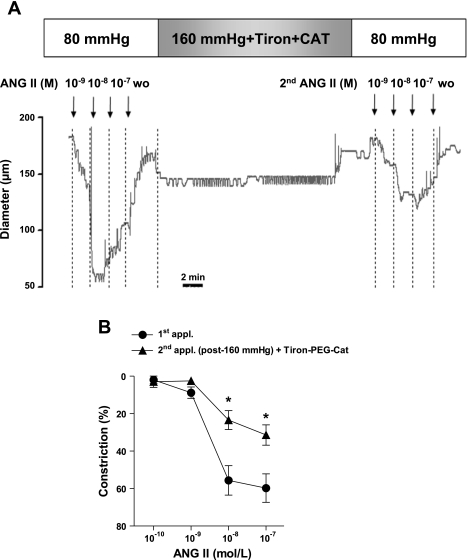
Previous studies have shown that high pressure, via oxidative stress, can affect several vascular signaling mechanisms. Thus, in this study, we have tested the hypothesis that high pressure-induced ROS, particularly H2O2 production, is responsible for the sustained Ang II-induced constrictions. To this end, arterioles were incubated with superoxide anion scavenger Tiron and PEG-catalase, and responses to sequential applications of Ang II were obtained in arterioles. If arterioles were exposed to normal 80 mmHg pressure, sequential applications of Ang II elicited diminished arteriolar constrictions in the presence of Tiron and PEG-catalase similar to the responses in the absence of ROS scavengers (max. constrictions to Ang II at 0.1 μmol/l: 1st application 58% ± 7% vs. 2nd application 28% ± 6%; P < 0.05). Original records and summary data show that presence of Tiron and PEG-catalase prevented the high pressure-induced augmentation of arteriolar constrictions to the second application of Ang II (Fig. 3). In contrast, constrictions to NE were not affected [max. constrictions to NE at 0.1 μmol/l: control 66% ± 4% vs. after H2O2 65% ± 4%; not significant (n.s.)]. These findings suggested the involvement of H2O2 in the high pressure-induced augmentation of Ang II-induced responses.
Fig. 3.
Original records (A) and summary data (n = 7; B) of constrictions of arterioles to repeated applications of Ang II (0.1 nmol/l to 0.1 μmol/l) exposed to 160 mmHg intraluminal pressure in the presence of reactive oxygen species scavenger Tiron and polyethylene glycol (PEG)-catalase (PEG-Cat). Data are means ± SE. *P < 0.05.
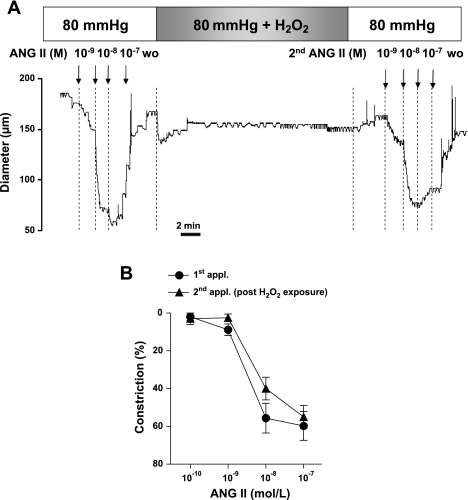
To confirm the specific role of H2O2, particularly the stable ROS, in the next series of experiments, in the presence of normal (80 mmHg) intraluminal pressure, Ang II-induced constrictions were obtained before and after the arterioles were transiently exposed to H2O2 (0.1 μmol/l for 30 min, Fig. 4). Whereas NE-induced constrictions were not affected by transient H2O2 exposure (max. constrictions to NE at 0.1 μmol/l: control 69% ± 8% vs. after H2O2 73% ± 3%; n.s.), exposure of the vessels to H2O2 augmented arteriolar constrictions to the second application to Ang II (Fig. 4).
Fig. 4.
Original records (A) and summary data (n = 7; B) of constrictions of arterioles to repeated applications of Ang II (0.1 nmol/l to 0.1 μmol/l) exposed to H2O2 (0.1 μM for 30 min) in the presence of 80 mmHg intraluminal pressure. Data are means ± SE.
DISCUSSION
Augmented vasomotor actions of Ang II have been thought to be responsible for the enhanced peripheral vascular resistance in various form of hypertension (2, 18, 27). Ang II, via activation of AT1 receptors, elicits constriction of resistance size arteries, which could lead to increased peripheral vascular resistance and hence systemic blood pressure. AT1 receptors possess a unique feature: on activation, they undergo rapid desensitization, becoming functionally unavailable for further stimulation, because they are internalized or located at the plasma membrane in an inactive state (12). This process reduces the number of active AT1 receptors available for further stimulation. This physiological mechanism, in a negative feedback manner, regulates the functional availability of AT1 receptors, thereby preventing sustained Ang II-mediated vascular signaling, such as increases in vascular tone and peripheral resistance (7). Under physiological conditions, sequential administration of Ang II elicits diminished vasoconstrictions. Present data confirm that, in the presence of normal levels of intraluminal pressure (80 mmHg for this size of arterioles), constrictions are substantially diminished on repeated administrations of Ang II. This phenomenon is also known as Ang II tachyphylaxis (13, 17, 24, 28). One of the novel findings of the present study is that this normal physiological function is greatly altered if arterioles are transiently exposed to high levels of intraluminal pressure (160 mmHg). Specifically, we have found that constrictions of isolated skeletal muscle arterioles to Ang II remained substantial on the second administration of Ang II if the vessels were exposed to high intraluminal pressure for 30 min. The results that constrictor responses to Ang II were not affected by selective inhibition of AT2 receptors suggest that high pressure specifically interferes with AT1 receptor-mediated arteriolar responses. Moreover, we have found that constrictions to the α-adrenergic agonist, NE, were similar in magnitude on repeated administrations and were unaffected by the high-pressure exposure of arterioles. These latter observations revealed a different behavior of AT1 and α-adrenergic receptors in pressure sensitivity on repeated agonist stimulation. These findings also suggest that mechanisms regulating downstream contractile function of arterioles are unlikely to be affected by acute high-pressure exposure.
In this study, we have also investigated the possible underlying mechanisms responsible for the high pressure-induced augmentation of Ang II-induced constrictions. It seems to be well-established that in hypertension vascular oxidative stress contributes to the development of cardiovascular dysfunction (2, 6). Despite extensive investigations, however, the role of ROS and their mechanism of action have not yet been fully elucidated in hypertension. It has been established that high intraluminal pressure itself elicits vascular oxidative stress (26, 27). In this context, recently we have demonstrated that acute increases in pressure from 80 to 160 mmHg for 30 min in isolated arterioles elicit increases in the level of NAD(P)H oxidase-derived superoxide anion and H2O2 in the vascular wall (26). These findings indicated that high intraluminal pressure itself alters vasomotor mechanisms intrinsic to vascular wall, in part, by inducing ROS production. Thus, in the present study, we hypothesized that high pressure induces ROS production, which is then responsible for the augmented Ang II-induced arteriolar constrictions. To this end, arterioles were incubated with ROS scavengers, and responses to sequential applications of Ang II were observed in arterioles exposed to normal or high intraluminal pressure. We have found that the presence of ROS scavengers prevented high pressure-induced augmentation of arteriolar constrictions to the second administration of Ang II (Fig. 4). In addition, ROS scavengers did not affect the magnitude of constrictions on repeated Ang II administration nor NE at a normal level of intraluminal pressure. We hypothesized a role for H2O2 in this process, and therefore Ang II-induced constrictions were also obtained after the arterioles were transiently exposed to H2O2. Whereas transient H2O2 exposure did not affect NE-induced constrictions, arteriolar constrictions to the second application of Ang II became augmented (Fig. 4). Collectively, these findings suggest that ROS, particularly H2O2, is involved in the high-pressure-induced augmentation of the Ang II-induced constrictions, whereas constrictor responses mediated by α-adrenergic receptors seem to be less sensitive to oxidative stress and high pressure. Of note is that H2O2 has been shown to activate MAPK in vascular smooth muscle cells (25), which may augment vasoconstriction by enhancing calcium sensitivity of the contractile elements. Indeed, Wolin et al. (30) describe that pulmonary arterial contraction to H2O2 can be reduced by MAPK inhibition. H2O2 can also inhibit the activity of both protein tyrosine phosphatases and serine/threonine phosphates via thiol oxidation of active site cysteine residues, a mechanism that has been shown to cause H2O2-dependent vasoconstriction (21, 23). Thus a possible effect of H2O2, other then affecting the availability of AT1 receptors, such as MAPK- and phosphatase-dependent regulation of calcium sensitivity, cannot be entirely excluded on the basis of present study.
The molecular mechanisms, by which high intraarteriolar pressure and H2O2 enhance the availability of AT1 receptors, could be an interesting subject of future studies, since the functional availability of AT1 receptors is regulated primarily by internalization, governed by complex mechanisms (8, 12). AT1 receptor internalization has been shown to involve both clathrin-dependent and -independent pathways (12), and it requires initial phosphorylation, hence acute desensitization of the AT1 receptors by G protein-coupled receptor kinases (1, 16). Thus it is possible that ROS interfere with the internalization and/or the dephosphorylation-dependent reactivation of AT1 receptors leading to sustained availability. Although these mechanisms have been described primarily in cell culture experiments, the functional evidence for the operation of these pathways in intact arterioles is still missing, most likely due to methodological difficulties. Nevertheless, future studies could elucidate which of these mechanisms are sensitive to the level of intraluminal pressure and affected by ROS and H2O2 and the possible contribution of these intracellular pathways mediating the pressure-sensitive enhancement of the surface availability of AT1 receptors.
Regardless of downstream signaling mechanisms, which need to be clarified, there are several important clinical implications of our present findings. It has been established that Ang II leads to vascular oxidative stress via increased activity of NAD(P)H oxidase (18). On the basis of the findings of the present study, we propose that high intraluminal pressure, via increased production of ROS, enhances the functional availability of AT1 receptors. Taken together, a pathological feed-forward mechanism can develop in the wall of resistance vessels, which could eventually lead to exaggerated Ang II-mediated increased vascular resistance and systemic blood pressure, without involvement of the systemic RAS. This feed-forward pathomechanism could initiate changes not only in the regulation of vasomotor function, but also in remodeling of the vascular wall. These ideas are suggested by findings of large clinical trials showing that many forms of human hypertension and consequent vascular diseases can be effectively treated with AT1 receptor blockers (3, 5, 22, 29) even in the absence of elevated levels of plasma Ang II.
In conclusion, we propose a novel pathophysiological mechanism by which high intraluminal pressure (independent of the circulating and tissue levels of Ang II), via increased production of H2O2, augments the functional availability of AT1 receptors, a mechanism that may operate in every form of hypertension or disease states associated with oxidative stress. Also, we propose that the level of intraluminal pressure, even in the physiological range, continuously modulates the functional availability of AT1 receptors and related signaling mechanisms.
GRANTS
This study was supported by the Hungarian Scientific Research Fund (OTKA) Grants F-048837 and T-048376, American Heart Association Affiliate Grants 0555897T, 0855910D and 0735540T, and National Heart, Lung, and Blood Institute Grant NHLB-43023. Zsolt Bagi holds a Bolyai Fellowship.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Anborgh PH, Seachrist JL, Dale LB, Ferguson SS. Receptor/beta-arrestin complex formation and the differential trafficking and resensitization of beta2-adrenergic and angiotensin II type 1A receptors. Mol Endocrinol 14: 2040–2053, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87: 840–844, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 359: 995–1003, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Fox KM Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet 362: 782–788, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Freel EM, Connell JM. Mechanisms of hypertension: the expanding role of aldosterone. J Am Soc Nephrol 15: 1993–2001, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Sympathoexcitation by central ANG II: roles for AT1 receptor upregulation and NAD(P)H oxidase in RVLM. Am J Physiol Heart Circ Physiol 288: H2271–H2279, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Gunther S, Gimbrone MA Jr, Alexander RW. Regulation by angiotensin II of its receptors in resistance blood vessels. Nature 287: 230–232, 1980. [DOI] [PubMed] [Google Scholar]
- 8.Hein L, Meinel L, Pratt RE, Dzau VJ, Kobilka BK. Intracellular trafficking of angiotensin II and its AT1 and AT2 receptors: evidence for selective sorting of receptor and ligand. Mol Endocrinol 11: 1266–1277, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Heistad DD, Lopez JA, Baumbach GL. Hemodynamic determinants of vascular changes in hypertension and atherosclerosis. Hypertension 17: III7–III11, 1991. [DOI] [PubMed] [Google Scholar]
- 10.Huang A, Koller A. Endothelin and prostaglandin H2 enhance arteriolar myogenic tone in hypertension. Hypertension 30: 1210–1215, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Huang A, Sun D, Koller A. Endothelial dysfunction augments myogenic arteriolar constriction in hypertension. Hypertension 22: 913–921, 1993. [DOI] [PubMed] [Google Scholar]
- 12.Hunyady L, Catt KJ, Clark AJ, Gaborik Z. Mechanisms and functions of AT(1) angiotensin receptor internalization. Regul Pept 91: 29–44, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Juul B, Aalkjaer C, Mulvany MJ. Responses of femoral resistance vessels to angiotensin in vitro. Eur J Pharmacol 135: 61–68, 1987. [DOI] [PubMed] [Google Scholar]
- 14.Koller A, Huang A. Development of nitric oxide and prostaglandin mediation of shear stress-induced arteriolar dilation with aging and hypertension. Hypertension 34: 1073–1079, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Koller A, Huang A. Impaired nitric oxide-mediated flow-induced dilation in arterioles of spontaneously hypertensive rats. Circ Res 74: 416–421, 1994. [DOI] [PubMed] [Google Scholar]
- 16.Lefkowitz RJ G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J Biol Chem 273: 18677–18680, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Linder AE, Thakali KM, Thompson JM, Watts SW, Webb RC, Leite R. Methyl-beta-cyclodextrin prevents angiotensin II-induced tachyphylactic contractile responses in rat aorta. J Pharmacol Exp Ther 323: 78–84, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 292: C82–C97, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Mulvany MJ Structure and function of small arteries in hypertension. J Hypertens Suppl 8: S225–S232, 1990. [PubMed] [Google Scholar]
- 20.Mulvany MJ, Aalkjaer C. Structure and function of small arteries. Physiol Rev 70: 921–961, 1990. [DOI] [PubMed] [Google Scholar]
- 21.Oeckler RA, Arcuino E, Ahmad M, Olson SC, Wolin MS. Cytosolic NADH redox and thiol oxidation regulate pulmonary arterial force through ERK MAP kinase. Am J Physiol Lung Cell Mol Physiol 288: L1017–L1025, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Price DA, Fisher ND. The renin-angiotensin system in blacks: active, passive, or what? Curr Hypertens Rep 5: 225–230, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Rhee SG, Kang SW, Jeong W, Chang TS, Yang KS, Woo HA. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol 17: 183–189, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Sorokin G, Grant NM, Egan BM, Lombard JH. Cyclooxygenase products do not modulate angiotensin II-induced contractions of human chorionic plate arteries. Am J Obstet Gynecol 167: 110–114, 1992. [DOI] [PubMed] [Google Scholar]
- 25.Thakali K, Davenport L, Fink GD, Watts SW. Cyclooxygenase, p38 mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase MAPK, Rho kinase, and Src mediate hydrogen peroxide-induced contraction of rat thoracic aorta and vena cava. J Pharmacol Exp Ther 320: 236–243, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Ungvari Z, Csiszar A, Huang A, Kaminski PM, Wolin MS, Koller A. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circulation 108: 1253–1258, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Ungvari Z, Csiszar A, Kaminski PM, Wolin MS, Koller A. Chronic high pressure-induced arterial oxidative stress: involvement of protein kinase C-dependent NAD(P)H oxidase and local renin-angiotensin system. Am J Pathol 165: 219–226, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vicaut E, Montalescot G, Hou X, Stucker O, Teisseire B. Arteriolar vasoconstriction and tachyphylaxis with intraarterial angiotensin II. Microvasc Res 37: 28–41, 1989. [DOI] [PubMed] [Google Scholar]
- 29.Weber M The telmisartan programme of research tO show Telmisartan End-organ proteCTION (PROTECTION) programme. J Hypertens Suppl 21: S37–S46, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Wolin MS, Gupte SA, Oeckler RA. Superoxide in the vascular system. J Vasc Res 39: 191–207, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 342: 145–153, 2000. [DOI] [PubMed] [Google Scholar]