Figure 3. Characterization of the B6.4−17/DMPC complexes at different L/P ratios.
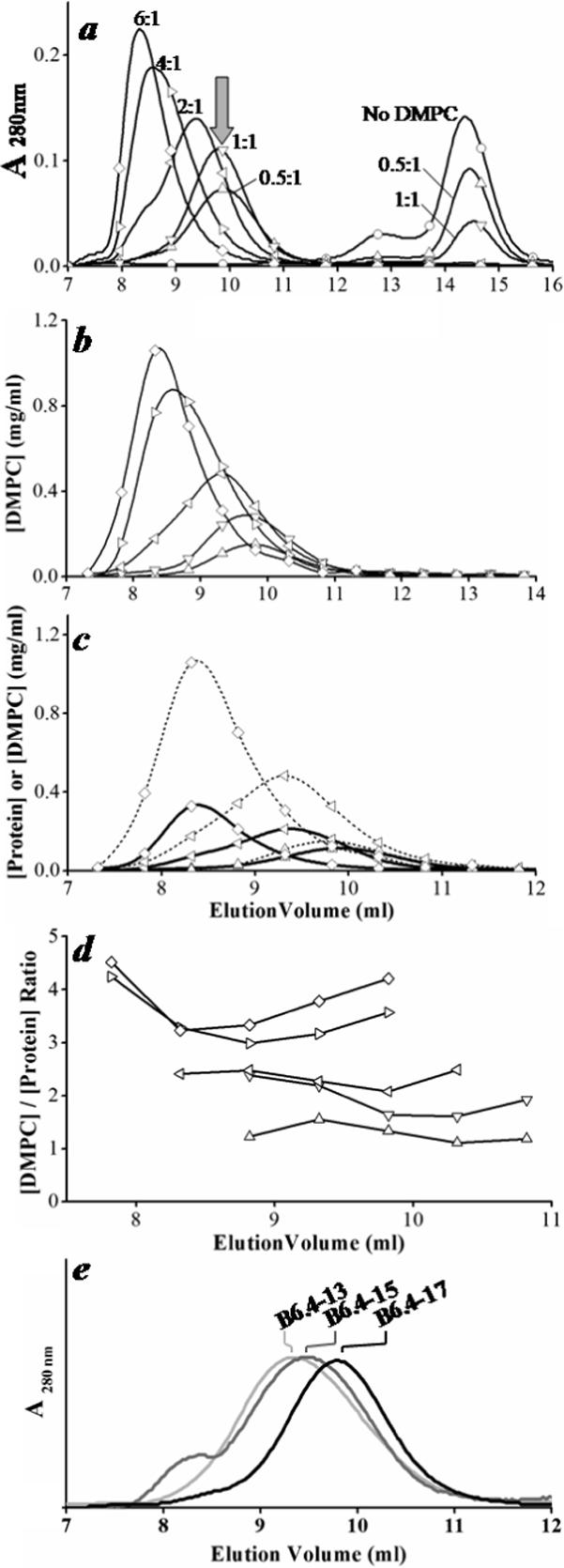
a. SEC analysis of B6.4−17 and its reconstituted DMPC particles prepared at different L/P ratios. B6.4−17 at 1 mg/ml was mixed with DMPC MLVs at 0:1 (○), 0.5:1 (△), 1:1 (▽), 2:1 (◁), 4:1 (▷) and 6:1 (◇) (wt:wt) L/P ratios in 0.6 ml TS buffer with 10 mM EDTA, 0.05% sodium azide. Samples were incubated at 24 °C overnight and analyzed on superdex GL 200 column. Arrow indicates the elution volume of a minimal-sized particle. The labeling scheme in a applies to b-d. b. DMPC concentration in the fractions collected from SEC. DMPC concentration is measured using the Bartlett assay (33). c. The overlay of the lipid and protein concentration. Protein concentration is calculated from UV absorbance at 280 nm. The elution volume of the protein profile has been corrected to match the void volume in the loops from the detector to the fraction collector. d. Actual L/P ratios in each fraction. Only fractions corresponding to the reconstituted particle peak are shown. e. The comparison of the minimal-sized particle prepared by B6.4−13, B6.4−15 and B6.4−17. All three proteins were prepared at 1 mg/ml protein concentration, 1:1 L/P ratios and analyzed by SEC as described previously. UV absorbance was normalized for better comparison.
