Abstract
Human herpesvirus 8 (HHV-8), or Kaposi's sarcoma (KS)-associated herpesvirus, has been linked to all forms of KS. The results of most current serological assays for the detection of HHV-8-specific antibodies have low levels of concordance among themselves. To establish a sensitive and specific testing strategy that can be used to screen for HHV-8-specific antibodies, three HHV-8 proteins, ORF65, ORF73, and K8.1A, were expressed by using baculoviral vectors in insect cells and incorporated into a monoclonal antibody-enhanced immunofluorescence assay (mIFA) termed the Sf9 three-antigen mIFA. The results obtained by this mIFA were compared to those obtained by a standard mIFA with an HHV-8-infected B-cell line (BC3 mIFA). Test sera were obtained from patients diagnosed with KS, human immunodeficiency virus type 1-infected patients at high risk for HHV-8 infection, and healthy controls from a local blood bank. The combined use of both assays had a sensitivity of 94% and a specificity of 96%. The performance of these two assays when they were used together indicates that they may be useful for the reliable detection of HHV-8-specific immunoglobulin G antibodies in a population.
Human herpesvirus 8 (HHV-8), also known as Kaposi's sarcoma (KS)-associated herpesvirus, is the latest human herpesvirus to be identified. It has been associated with all four clinical presentations of KS (the classic, endemic, AIDS-related, and iatrogenic forms) (6, 15). HHV-8 has also been detected in patients with primary effusion B-cell lymphomas (PELs) and multicentric Castleman's disease (MCD) (4, 30).
In the general population, the seroprevalence of HHV-8 shows marked geographical variations. HHV-8 infection is endemic in Africa and the Mediterranean region, and in areas where it is not endemic, it is found at a higher prevalence in homosexual men and immunosuppressed individuals (8, 9, 13, 24). Its routes of transmission are still not well understood, but both horizontal transmission and vertical transmission are possible (2, 10, 21). Horizontal transmission can occur by sexual and nonsexual routes. HHV-8 seroconversion is observed during adulthood among individuals in most developed countries, most likely due to sexual transmission, and occurs in childhood in areas of endemicity, most likely due to nonsexual horizontal transmission. HHV-8 DNA has been detected in saliva, making saliva a potential source of transmission via close interpersonal contact (1-3, 9, 18, 22, 31). HHV-8 DNA cannot be detected in all infected individuals; therefore, serology is the method of choice used in epidemiological studies to screen for infected individuals.
The development of high-performance serologic tests has been achieved only to a limited degree due to an incomplete understanding of the known immunodominant proteins, a lack of well-characterized uninfected and infected individuals who may serve as controls, and reported wide variations in antibody titers among infected individuals. While various serological assays have been shown to have variable performance characteristics and concordance, immunofluorescence assays (IFAs) have been considered one of the most sensitive assays for the detection of antibodies against HHV-8 (11, 23, 27). IFA was one of the first assays to be used for the detection of HHV-8 antibodies (20). Cell lines derived from patients with PELs and individuals chronically infected with HHV-8, which mainly express latent and low levels of lytic antigens, have been used for latent or lytic antgen IFAs. The level of lytic antigens can be increased by induction with tetradecanoyl phorbol acetate. By the use of sera from KS patients, several proteins have been identified to be highly reactive antigens. These include open reading frame (ORF) proteins 6, 8, 9, 25, 26, 39, 59, 65, 68, and 73; K8.1A; and K8.1B (5). Of these proteins, ORF59, K8.1A, ORF65, and ORF73 have been used in the development of various enzyme immunoassays (EIAs) and have been reported to be good candidate antigens (5, 14, 16, 19, 28, 32). There are now two commercially available EIAs that use whole-virus lysate and synthetic peptides.
Here we report on the use of a screening strategy for the detection of HHV-8-specific antibodies in plasma samples. An IFA with Sf9 cells expressing predominant proteins encoded by HHV-8 (ORF65, ORF73, K8.1) was used in conjunction with an IFA that uses stimulated BC3 cells to obtain a sensitive and specific testing strategy.
MATERIALS AND METHODS
Cell culture.
BC3 cells (ATCC) were grown in RPMI 1640 medium supplemented with 20% fetal calf serum, l-glutamine, sodium pyruvate, HEPES, and d-glucose. Sf9 insect cells were maintained as a suspension culture in SF 900 II medium (Invitrogen) supplemented with 10% fetal calf serum and 1% gentamicin.
Patient sera.
A total of 219 samples were used in this study. Of these, 108 samples were collected from patients visiting the Adult Oncology Unit at the University of Miami Miller School of Medicine. Two plasma samples were collected from KS patients at the University Teaching Hospital, Lusaka, Zambia, as a part of an ongoing study that is investigating HHV-8 transmission within families. Blood banks in Lincoln, NE, and Kansas City, KS, contributed 109 plasma samples. The ethics committee of the Institutional Review Board at the University of Nebraska approved the study. All samples were coded and screened without knowledge of the identity of the patient or the diagnosis. Subsequently, patient serum samples were divided into three groups. Group A consisted of a total of 33 samples collected from histologically identified KS patients. This group also included samples from one patient with PEL and one patient with MCD. Group B (the high-risk group) consisted of 77 samples collected from human immunodeficiency virus type 1 (HIV-1)-positive patients with other cancers (not KS, PEL, or MCD). Group C (blood bank donors) consisted of 109 samples collected from healthy blood bank donors with low-risk lifestyle behaviors.
Preparation of BC3 cell slides.
BC3 cells at a concentration of 7 × 105 per ml were treated with tetradecanoyl phorbol acetate at a final concentration of 20 ng/ml for 72 h. The cells were fixed in 4% paraformaldehyde for 20 min at room temperature, washed with phosphate-buffered saline (PBS), and permeabilized with 0.1% Triton X-100 for 15 min at room temperature. The cells were washed and resuspended in PBS. Approximately 10,000 cells were spotted per well onto 12-well Teflon-coated slides (Electron Microscopy Sciences) and stored at −80°C.
Preparation of Sf9 cell slides expressing HHV-8 antigens.
Recombinant baculoviruses expressing glutathione S-transferase (GST)-tagged lytic proteins ORF65 and K8.1A and latent protein ORF73 (provided by Bala Chandran, Rosalind Franklin University of Medicine and Science, Chicago, IL) were used to develop the Sf9 cell monoclonal antibody-enhanced IFAs (mIFAs). Baculovirus-infected Sf9 cells expressing GST alone were used as a negative control to detect background and nonspecific fluorescence. The titers of all baculovirus stocks were determined, and infections were initiated separately with the three baculovirus stocks, each of which expressed one recombinant HHV-8 protein at a multiplicity of infection of 10. Infected cells were monitored daily for viability and cell diameter by using a Vi-Cell counter (Beckman-Coulter). The expression of each protein was evaluated by Western blot analysis with anti-GST antibody (Santa Cruz Biotechnology) following sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Fig. 1). At 72 h postinfection, the cells were harvested, mixed in an equal ratio (1:1:1) of viable cells, and subsequently fixed by the BC3 cell method. This was called the “Sf9 three-antigen” test. All fixed slides were stored at −80°C.
FIG. 1.
Western blot analyses of ORF65, ORF73, and K8.1A proteins expressed in Sf9 insect cells. Infected cells were harvested at 72 h postinfection and were lysed by sonication. Specific proteins were detected by using anti-GST antibodies. Lanes 1 to 3, ORF65 (48 kDa), ORF73 (>150 kDa), and K8.1A (52 kDa), respectively. Arrows indicate the expressed proteins.
A modification of the method was also used. At 72 h postinfection, Sf9 cells infected with baculovirus expressing the ORF73, ORF65, or K8.1 antigen were harvested, fixed, and spotted individually on separate slides. This method was used to screen patients for the presence of latent or lytic antibodies and is referred to as the “single-antigen Sf9 assay.”
mIFAs.
All serum samples were diluted 1:40 and centrifuged at high speed for 1 min immediately before they were used. All slides were warmed to room temperature, individual serum samples were applied to each well, and the slides were incubated at 37°C for 30 min in a humidified chamber. The slides were washed (six times) with PBS and were then incubated with mouse anti-human immunoglobulin G monoclonal antibody at 37°C for 30 min. The slides were again washed with PBS and then incubated with goat anti-mouse Cy2-conjugated antibody (Jackson Laboratories) at 37°C for 30 min. After the cells were washed, they were stained with 0.004% Evans blue for 5 min, washed, and mounted. The mIFA procedure was the same for BC3 and Sf9 cell slides.
Criteria for being HHV-8 seropositive.
All slides were read by two independent readers without knowledge of the patient's identity, the clinical diagnosis, the patient's HIV-1 status, or the other reader's results. To reduce subjectivity in observing the specific fluorescence, the slides were read independently by two experienced laboratory workers. A sample was considered positive if specific fluorescence was observed by both readers. In the case of discordant results, the assay was repeated. On repetition, if discordant results were again obtained, then these patients were considered seronegative. All samples determined to be positive by the BC3 mIFA and the Sf9 three-antigen assay were considered positive. If a patient was positive by just one assay or negative by both assays, he or she was considered HHV-8 negative. This testing scheme is summarized in Fig. 2.
FIG. 2.
Scheme used to screen patients for antibodies against HHV-8 and determine their serological status.
Statistical analysis.
All data were entered into and analyzed with the SPSS program (version 15). The kappa statistic was computed to determine the concordance between the standard BC3 mIFA and the Sf9 mIFA. The sensitivity of detection of HHV-8-specific antibodies was calculated as the number of true-positive samples/(number of true-positive samples + number of false-negative samples). All serum samples collected from reliably diagnosed cases of KS, PEL, and MCD and found to be serologically positive by both assays were considered true positives. The results were considered false negative if serum samples collected from these patients were found to be negative by our criteria.
Specificity was calculated as the number of true-negative samples/(number of true-negative samples + number of false-positive samples). All serum samples collected from healthy blood donors and found to be serologically negative by either assay were considered true negative. The results were considered false positive if samples collected from these patients were found to be positive.
RESULTS
HHV-8 seroprevalence.
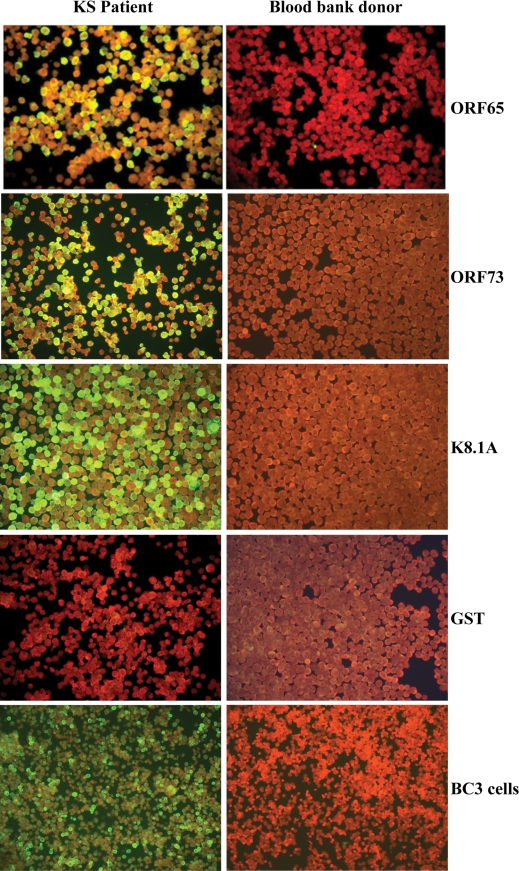
All serum samples were assigned a unique identification number and were screened blinded by both mIFAs. Representative mIFA images for a positive and a negative patient are shown in Fig. 3. After serological screening was completed, the specimens were divided into three groups on the basis of the patient's clinical diagnosis, as described in the Materials and Methods section (Table 1). By using the strategy described in Fig. 2, the overall seroprevalence in group A (KS, PEL, and MCD patients) was 93.9%. Two samples were considered seronegative, including one sample which tested positive by the BC3 mIFA but negative by the Sf9 three-antigen mIFA and a second sample that was not positive by either of the two assays. The seroprevalence in group B (the high-risk group) was 58.4%, with 45 of 77 samples being positive by both assays. The remaining 32 samples were considered seronegative. Fifteen of these 32 seronegative samples (19.5%) were negative by both assays. Nine (11.7%) and eight (10.4%) samples were positive by the BC3 mIFA and the Sf9 three-antigen assay alone, respectively. Both assays detected HHV-8-specific antibodies in only four (3.6%) of the patients in group C (blood bank donors). The remaining 105 samples were considered seronegative. Of these 105 seronegative samples, 2 (1.8%) and 7 (6.4%) were positive by the BC3 mIFA and the Sf9 three-antigen assay alone, respectively.
FIG. 3.
Representative staining patterns of mIFAs of ORF65, ORF73, and K8.1A proteins expressed in Sf9 insect cells and BC3 cells by using positive sera (from patients with KS; left column) and negative sera (from blood bank donors; right column).
TABLE 1.
Seroprevalence of HHV-8-specific antibodies in positive control group, high-risk group, and blood bank donors
| HHV-8 status and assay resulta | No. (%) of samples
|
||
|---|---|---|---|
| Positive group | High-risk group | Negative group | |
| HHV-8 positive, BC3+/Sf9 three-antigen+ | 31 (94.0) | 45 (58.4) | 4 (3.7) |
| HHV-8 negative | |||
| BC3+/Sf9 three-antigen− | 1 (3.0) | 9 (11.7) | 2 (1.8) |
| BC3−/Sf9 three-antigen+ | 0 | 8 (10.4) | 7 (6.4) |
| BC3−/Sf9 three-antigen− | 1 (3.0) | 15 (19.5) | 96 (88.1) |
A positive sign refers to patients who tested positive by that assay, and a negative sign refers to patients who tested negative by that assay.
Latent and lytic antibody profiles.
All samples that were positive by the Sf9 three-antigen assay were screened by the single-antigen Sf9 method to ascertain the latent and lytic antibody profiles present in different groups. Also, we compared the antibody profiles of those samples that were positive by both the BC3 mIFA and the Sf9 three-antigen assay to those of samples that were BC3 mIFA negative and Sf9 three-antigen assay positive (Table 2).
TABLE 2.
Antibody profiles of all Sf9 three-antigen-positive samples against latent (ORF73) and lytic (ORF65 or K8.1) antigensa
| Latent antigen profile | Lytic antigen profile | No. of samples
|
|||||
|---|---|---|---|---|---|---|---|
| Positive group
|
High-risk group
|
Negative group
|
|||||
| BC3+ | BC3− | BC3+ | BC3− | BC3+ | BC3− | ||
| + | + | 23 | 0 | 29 | 6 | 1 | 1 |
| + | − | 2 | 0 | 8 | 1 | 0 | 0 |
| − | + | 6 | 0 | 8 | 1 | 3 | 6 |
| Total | 31 | 0 | 45 | 8 | 4 | 7 | |
A positive sign refers to patients who tested positive by that assay, and a negative sign refers to patients who tested negative by that assay.
BC3 mIFA- and Sf9 three-antigen assay-positive samples.
When the mIFAs were performed with the ORF65, K8.1, and ORF73 antigens separately, 74% (23/31) of the Sf9 three-antigen mIFA-positive and BC3 mIFA-positive samples in group A reacted to both the latent (ORF73) and the lytic (ORF65 or K8.1) antigens. Two samples had detectable antibodies against latent antigen only, and six samples reacted to at least one lytic antigen. In group B, 64% (29/45) of the seropositive samples reacted to both the latent and the lytic antigens. Eight samples each reacted only to either the latent antigen or the lytic antigen. In group C, only one of four seropositive samples was positive for both the latent and the lytic antigens. Three samples were positive for lytic antigens only.
BC3 mIFA-negative and Sf9 three-antigen assay-positive samples.
We also wanted to compare the antibody profiles of patients who were negative by the BC3 mIFA described above. We did not observe any such sample in group A, but there were six such samples in group B. All these six samples were negative by the BC3 mIFA and had detectable titers to both the latent and the lytic antigens, as observed by using the Sf9 three-antigen assay. In the same group, one sample each was positive for the latent or the lytic antigen only. In group C, we observed that only one sample was positive for both the latent and the lytic antigens but that six samples reacted positively to the lytic antigens.
Concordance, assay sensitivity, and assay specificity.
The kappa value, which denotes the concordance of the results of the BC3 mIFA and the Sf9 three-antigen assay, was calculated. The overall kappa value for all 219 samples was 0.75. The sensitivity of the screening strategy was then evaluated by using group A (positive group) samples and was calculated as described in the Materials and Methods section. The sensitivity of detection of positive samples by this combined strategy was 93.9%. The specificity was calculated by using samples from group C (blood bank donors). This yielded a specificity of 96.3%.
DISCUSSION
A large proportion of infected but asymptomatic individuals do not have detectable HHV-8 DNA in their peripheral blood; therefore, serology is a more reliable method for the identification of HHV-8 infection. However, a major hurdle in obtaining clear data on HHV-8 seroprevalence in a population, understanding the route of transmission, and routine monitoring of at-risk individuals is the lack of a reliable assay that can detect antibodies in human sera.
Most laboratories use in-house assays, which have various levels of sensitivity, specificity, and concordance of results, to screen for HHV-8-specific antibodies (23, 26, 27). Currently, no assay is clearly superior because of its sensitivity and specificity. Our goal was to develop a sensitive and specific Sf9 three-antigen IFA that is cost-effective and that can be used as a confirmatory test to validate IFA results by using HHV-8-infected cells. In the study described in this report, both the BC3 assay and the Sf9 three-antigen assay were used in parallel to investigate the concordance of results of the two assays. Given the findings presented in this report, the Sf9 three-antigen assay can be used in tandem as a confirmatory assay with the BC3 assay for screening for HHV-8-specific antibodies. We used samples from well-characterized patients to evaluate the sensitivity and specificity of this testing strategy. Both the BC3 and the Sf9 three-antigen mIFAs are designed to detect HHV-8-specific immunoglobulin G antibodies against latent and lytic antigens. ORF73 is the major latent protein and encodes the latency-associated nuclear antigen. ORF65 is a lytic-phase protein and encodes the minor capsid antigen. It has been reported to be one of the immunodominant antigens that can be used for a sensitive serological assay (17). The Sf9 three-antigen assay is flexible because it can be expanded to incorporate more antigens, if needed. Overexpression of the immunodominant proteins also helps to easily identify patients with very low HHV-8-specific antibody titers. Each antigen can be expressed individually in Sf9 cells to monitor the antibody responses to latent and lytic antigens separately. More importantly, GST-expressing Sf9 cells served as good negative controls and were used to check for nonspecific binding and fluorescence. This is one of the major drawbacks of using BC3 cells, which lack matched negative control cells. Background or nonspecific fluorescence, which is commonly found in IFAs, cannot be ruled out when BC3 or other HHV-8-harboring cell lines are used. Populations which have been shown to produce this nonspecific reactivity include serum samples from individuals with high numbers of sexual partners, patients with parasitic infections, and people exposed to a high number of pathogens. Residue, sediments, or high lipid contents can also cause high background fluorescence and was controlled in our assay by the centrifugation of each sample immediately before it was used (7).
The criterion that we used to consider a patient HHV-8 positive in our study was based on the results of two IFAs. A patient was considered positive if the plasma sample gave specific fluorescence at a dilution of 1:40 by both IFAs. While this strategy increases the specificity of detection of HHV-8-specific antibodies, it may decrease the sensitivity of detection. By using this testing strategy we could detect HHV-8-specific antibodies in 94% of clinically diagnosed cases of KS, PEL, and MCD. Among the positive patients with KS, one patient was negative by both the BC3 and the Sf9 three-antigen mIFAs. This patient had undergone antiretroviral treatment after the development of KS; the treatment could have suppressed the viral load and led to the complete seroreversion for HHV-8-specific antibodies by the time of the present study. Seroreversion has, in fact, been documented in patients after treatment and the regression of symptoms (25). CD4+ cell count data were not available to help gauge the level of immunosuppression, which may also help explain the unexpected HHV-8 seronegativity. It is possible that the sensitivities of both assays were too low to detect the very low antibody titer in this patient. One sample in the positive group was positive by the BC3 mIFA but negative by the Sf9 mIFA. This could be because nonspecific fluorescence was shown by the BC3 mIFA, but this cannot be reliably confirmed because of a lack of a matched negative cell line. It is also likely that for this patient, none of the three antigens that were expressed in Sf9 cells elicited an immune response, which further underscores the importance of using multiple antigens for screening. This suggests that there is a need for the identification and incorporation of more antigens for the routine screening of patients for HHV-8.
We had chosen the blood bank donor group as our truly negative controls because blood donors are selected for minimal behavioral risk. Using this testing scheme, we found a seroprevalence of 3.7% in the blood donor group. This is not surprising, because a low HHV-8 seroprevalence of about 3% has been reported in U.S. blood donors (23). We observed a similar seroprevalence in the blood bank donors, which we had assumed were negative. In our case, it reduced the specificity (96%) of this testing strategy. The lack of a “gold standard” serological assay that can reliably identify patients who are truly infected versus those who are uninfected makes it difficult to test the accuracy of the assays currently in use.
Group B patients were included as a test group. The HHV-8 seroprevalence in samples from group B was within the expected range. HIV-1-infected patients from North America have been reported to have an HHV-8 seroprevalence in the range of 20 to 50% (12, 20, 29). We observed a similar seroprevalence rate of 58.4% in this group. The antibody profile showed that 82% of the seropositive samples had lytic antibodies, which is indicative of active replication and which has been shown to be a risk factor for the development of KS. No follow-up was available for these patients, and we do not know if any of them developed KS.
This assay could have some other drawbacks. It is more labor-intensive than EIA, and reading of the slides can be subjective. We tried to reduce the subjectivity by employing two readers who read all the slides independently. EIAs have frequently employed KS patients to obtain cutoff values for optical density. This approach might exclude asymptomatic individuals who are seropositive and frequently have very low titers of HHV-8-specific antibodies. This method has proven to be sensitive in detecting HHV-8-specific antibodies from asymptomatic children and adults in Zambia, a region where HHV-8 infection is endemic (21a). In our experience, most asymptomatic individuals in this cohort in Zambia have very low antibody titers, and the use of EIAs with high cutoff values is not suitable for such epidemiological studies. We believe that our stringent criterion for the detection of seropositive cases may still be underestimating the number of seropositive cases and reducing the level of sensitivity. However, this scheme increases the specificity of detection of HHV-8-specific antibodies by excluding false-positive results. For this study, we did not test these patient samples for other known ubiquitous herpesviruses, such as cytomegalovirus, Epstein-Barr virus, and herpes simplex virus type 1. Our observations for other adult patient samples from similar locations have shown a very high seroprevalence.
A reliable serological assay for HHV-8 could be a useful tool for the accurate monitoring and diagnosis of HHV-8 infection. In the absence of a gold standard, this strategy has proven to be helpful in conducting seroepidemiological studies in an area of endemicity. A greater understanding of the HHV-8 antibody response is required to perfect the current serological testing strategy. In conclusion, we describe a new serological approach that can be used to screen patients for the presence of HHV-8-specific antibodies and that is sensitive and specific and reduces the chance of detection of false-positive results. Finally, further refinement of this approach to incorporate more antigens is ongoing.
Acknowledgments
We gratefully acknowledge the contribution of Bala Chandran for the baculovirus constructs and Clinton Jones for providing us with Sf9 cells. We thank Hui-Ju Wen for technical help.
This work was supported in part by PHS grants RO1 CA75903 and RO1 CA082274, Fogarty International Training grants D43 TW01492 and T32 A1060547, and NCRR COBRE grant P20 RR15635 to C.W. T.J.M. and S.P. were Fogarty fellows. K.L.C. is supported by the Ruth L. Kirschstein National Research Service Award from the National Institute of Allergy and Infectious Diseases. L.N.C. and K.L.C. were supported by the INBRE program P20 RR016469 of the National Center for Research Resources.
Footnotes
Published ahead of print on 28 May 2008.
REFERENCES
- 1.Blackbourn, D. J., and J. A. Levy. 1997. Human herpesvirus 8 in semen and prostate. AIDS 11:249-250. [DOI] [PubMed] [Google Scholar]
- 2.Brayfield, B. P., C. Kankasa, J. T. West, J. Muyanga, G. Bhat, W. Klaskala, C. D. Mitchell, and C. Wood. 2004. Distribution of Kaposi sarcoma-associated herpesvirus/human herpesvirus 8 in maternal saliva and breast milk in Zambia: implications for transmission. J. Infect. Dis. 189:2260-2270. [DOI] [PubMed] [Google Scholar]
- 3.Casper, C., E. Krantz, S. Selke, S. R. Kuntz, J. Wang, M. L. Huang, J. S. Pauk, L. Corey, and A. Wald. 2007. Frequent and asymptomatic oropharyngeal shedding of human herpesvirus 8 among immunocompetent men. J. Infect. Dis. 195:30-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 5.Chandran, B., M. S. Smith, D. M. Koelle, L. Corey, R. Horvat, and E. Goldstein. 1998. Reactivities of human sera with human herpesvirus-8-infected BCBL-1 cells and identification of HHV-8-specific proteins and glycoproteins and the encoding cDNAs. Virology 243:208-217. [DOI] [PubMed] [Google Scholar]
- 6.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 7.Chatlynne, L. G., and D. V. Ablashi. 1999. Seroepidemiology of Kaposi's sarcoma-associated herpesvirus (KSHV). Semin. Cancer Biol. 9:175-185. [DOI] [PubMed] [Google Scholar]
- 8.Chatlynne, L. G., W. Lapps, M. Handy, Y. Q. Huang, R. Masood, A. S. Hamilton, J. W. Said, H. P. Koeffler, M. H. Kaplan, A. Friedman-Kien, P. S. Gill, J. E. Whitman, and D. V. Ablashi. 1998. Detection and titration of human herpesvirus-8-specific antibodies in sera from blood donors, acquired immunodeficiency syndrome patients, and Kaposi's sarcoma patients using a whole virus enzyme-linked immunosorbent assay. Blood 92:53-58. [PubMed] [Google Scholar]
- 9.Dedicoat, M., and R. Newton. 2003. Review of the distribution of Kaposi's sarcoma-associated herpesvirus (KSHV) in Africa in relation to the incidence of Kaposi's sarcoma. Br. J. Cancer 88:1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dedicoat, M., R. Newton, K. R. Alkharsah, J. Sheldon, I. Szabados, B. Ndlovu, T. Page, D. Casabonne, C. F. Gilks, S. A. Cassol, D. Whitby, and T. F. Schulz. 2004. Mother-to-child transmission of human herpesvirus-8 in South Africa. J. Infect. Dis. 190:1068-1075. [DOI] [PubMed] [Google Scholar]
- 11.Engels, E. A., M. D. Sinclair, R. J. Biggar, D. Whitby, P. Ebbesen, J. J. Goedert, and J. L. Gastwirth. 2000. Latent class analysis of human herpesvirus 8 assay performance and infection prevalence in sub-Saharan Africa and Malta. Int. J. Cancer 88:1003-1008. [DOI] [PubMed] [Google Scholar]
- 12.Gao, S. J., L. Kingsley, M. Li, W. Zheng, C. Parravicini, J. Ziegler, R. Newton, C. R. Rinaldo, A. Saah, J. Phair, R. Detels, Y. Chang, and P. S. Moore. 1996. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi's sarcoma. Nat. Med. 2:925-928. [DOI] [PubMed] [Google Scholar]
- 13.Gessain, A., P. Mauclere, M. van Beveren, S. Plancoulaine, A. Ayouba, J. L. Essame-Oyono, P. M. Martin, and G. de The. 1999. Human herpesvirus 8 primary infection occurs during childhood in Cameroon, Central Africa. Int. J. Cancer 81:189-192. [DOI] [PubMed] [Google Scholar]
- 14.He, F., X. Wang, B. He, Z. Feng, X. Lu, Y. Zhang, S. Zhao, R. Lin, Y. Hui, Y. Bao, Z. Zhang, and H. Wen. 2007. Human herpesvirus 8: serovprevalence and correlates in tumor patients from Xinjiang, China. J. Med. Virol. 79:161-166. [DOI] [PubMed] [Google Scholar]
- 15.Huang, Y. Q., J. J. Li, M. H. Kaplan, B. Poiesz, E. Katabira, W. C. Zhang, D. Feiner, and A. E. Friedman-Kien. 1995. Human herpesvirus-like nucleic acid in various forms of Kaposi's sarcoma. Lancet 345:759-761. [DOI] [PubMed] [Google Scholar]
- 16.Katano, H., T. Iwasaki, N. Baba, M. Terai, S. Mori, A. Iwamoto, T. Kurata, and T. Sata. 2000. Identification of antigenic proteins encoded by human herpesvirus 8 and seroprevalence in the general population and among patients with and without Kaposi's sarcoma. J. Virol. 74:3478-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katano, H., T. Sata, T. Suda, T. Nakamura, N. Tachikawa, H. Nishizumi, S. Sakurada, Y. Hayashi, M. Koike, A. Iwamoto, T. Kurata, and S. Mori. 1999. Expression and antigenicity of human herpesvirus 8 encoded ORF59 protein in AIDS-associated Kaposi's sarcoma. J. Med. Virol. 59:346-355. [DOI] [PubMed] [Google Scholar]
- 18.Koelle, D. M., M. L. Huang, B. Chandran, J. Vieira, M. Piepkorn, and L. Corey. 1997. Frequent detection of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) DNA in saliva of human immunodeficiency virus-infected men: clinical and immunologic correlates. J. Infect. Dis. 176:94-102. [DOI] [PubMed] [Google Scholar]
- 19.Laney, A. S., S. C. Dollard, H. W. Jaffe, M. K. Offermann, T. J. Spira, C. J. Gunthel, P. E. Pellett, and M. J. Cannon. 2004. Repeated measures study of human herpesvirus 8 (HHV-8) DNA and antibodies in men seropositive for both HHV-8 and HIV. AIDS 18:1819-1826. [DOI] [PubMed] [Google Scholar]
- 20.Lennette, E. T., D. J. Blackbourn, and J. A. Levy. 1996. Antibodies to human herpesvirus type 8 in the general population and in Kaposi's sarcoma patients. Lancet 348:858-861. [DOI] [PubMed] [Google Scholar]
- 21.Mantina, H., C. Kankasa, W. Klaskala, B. Brayfield, J. Campbell, Q. Du, G. Bhat, F. Kasolo, C. Mitchell, and C. Wood. 2001. Vertical transmission of Kaposi's sarcoma-associated herpesvirus. Int. J. Cancer 94:749-752. [DOI] [PubMed] [Google Scholar]
- 21a.Minhas, V., K. L. Crabtree, A. Chao, T. J. M'soka, C. Kankasa, M. Bulterys, C. D. Mitchell, and C. Wood. Early childhood infection by human herpesvirus 8 in Zambia and the role of human immunodeficiency virus type 1 coinfection in a highly endemic area. Am. J. Epidemiol., in press. [DOI] [PMC free article] [PubMed]
- 22.Pauk, J., M. L. Huang, S. J. Brodie, A. Wald, D. M. Koelle, T. Schacker, C. Celum, S. Selke, and L. Corey. 2000. Mucosal shedding of human herpesvirus 8 in men. N. Engl. J. Med. 343:1369-1377. [DOI] [PubMed] [Google Scholar]
- 23.Pellett, P. E., D. J. Wright, E. A. Engels, D. V. Ablashi, S. C. Dollard, B. Forghani, S. A. Glynn, J. J. Goedert, F. J. Jenkins, T. H. Lee, F. Neipel, D. S. Todd, D. Whitby, G. J. Nemo, and M. P. Busch. 2003. Multicenter comparison of serologic assays and estimation of human herpesvirus 8 seroprevalence among US blood donors. Transfusion 43:1260-1268. [DOI] [PubMed] [Google Scholar]
- 24.Plancoulaine, S., L. Abel, M. van Beveren, D. A. Tregouet, M. Joubert, P. Tortevoye, G. de The, and A. Gessain. 2000. Human herpesvirus 8 transmission from mother to child and between siblings in an endemic population. Lancet 356:1062-1065. [DOI] [PubMed] [Google Scholar]
- 25.Quinlivan, E. B., R. X. Wang, P. W. Stewart, C. Kolmoltri, N. Regamey, P. Erb, and P. L. Vernazza. 2001. Longitudinal sero-reactivity to human herpesvirus 8 (KSHV) in the Swiss HIV Cohort 4.7 years before KS. J. Med. Virol. 64:157-166. [DOI] [PubMed] [Google Scholar]
- 26.Rabkin, C. S., T. F. Schulz, D. Whitby, E. T. Lennette, L. I. Magpantay, L. Chatlynne, R. J. Biggar, et al. 1998. Interassay correlation of human herpesvirus 8 serologic tests. J. Infect. Dis. 178:304-309. [DOI] [PubMed] [Google Scholar]
- 27.Schatz, O., P. Monini, R. Bugarini, F. Neipel, T. F. Schulz, M. Andreoni, P. Erb, M. Eggers, J. Haas, S. Butto, M. Lukwiya, J. R. Bogner, S. Yaguboglu, J. Sheldon, L. Sarmati, F. D. Goebel, R. Hintermaier, G. Enders, N. Regamey, M. Wernli, M. Sturzl, G. Rezza, and B. Ensoli. 2001. Kaposi's sarcoma-associated herpesvirus serology in Europe and Uganda: multicentre study with multiple and novel assays. J. Med. Virol. 65:123-132. [PubMed] [Google Scholar]
- 28.Sergerie, Y., Y. Abed, J. Roy, and G. Boivin. 2004. Comparative evaluation of three serological methods for detection of human herpesvirus 8-specific antibodies in Canadian allogeneic stem cell transplant recipients. J. Clin. Microbiol. 42:2663-2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simpson, G. R., T. F. Schulz, D. Whitby, P. M. Cook, C. Boshoff, L. Rainbow, M. R. Howard, S. J. Gao, R. A. Bohenzky, P. Simmonds, C. Lee, A. de Ruiter, A. Hatzakis, R. S. Tedder, I. V. Weller, R. A. Weiss, and P. S. Moore. 1996. Prevalence of Kaposi's sarcoma associated herpesvirus infection measured by antibodies to recombinant capsid protein and latent immunofluorescence antigen. Lancet 348:1133-1138. [DOI] [PubMed] [Google Scholar]
- 30.Soulier, J., L. Grollet, E. Oksenhendler, P. Cacoub, D. Cazals-Hatem, P. Babinet, M. F. d'Agay, J. P. Clauvel, M. Raphael, L. Degos, et al. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276-1280. [PubMed] [Google Scholar]
- 31.Vieira, J., M. L. Huang, D. M. Koelle, and L. Corey. 1997. Transmissible Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in saliva of men with a history of Kaposi's sarcoma. J. Virol. 71:7083-7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu, L., R. Wang, A. Sweat, E. Goldstein, R. Horvat, and B. Chandran. 1999. Comparison of human sera reactivities in immunoblots with recombinant human herpesvirus (HHV)-8 proteins associated with the latent (ORF73) and lytic (ORFs 65, K8.1A, and K8.1B) replicative cycles and in immunofluorescence assays with HHV-8-infected BCBL-1 cells. Virology 256:381-392. [DOI] [PubMed] [Google Scholar]