Abstract
It is generally believed that probiotic bacteria need to survive gastrointestinal transit to exert a health-promoting effect. In this study, a genuine luxS mutant and a luxS mutant containing unknown suppressor mutations of the probiotic strain Lactobacillus rhamnosus GG were compared to the wild type for survival and persistence in the murine gastrointestinal tract. The LuxS enzyme, catalyzing the production of the autoinducer-2 signaling molecule, also forms an integral part of the activated methyl cycle and the metabolism of methionine and cysteine. The genuine luxS mutant CMPG5412 showed drastically reduced persistence in mice, which was related to less survival in simulated gastric juice, indicating that LuxS metabolism is crucial for the gastric stress resistance of L. rhamnosus GG. The suppressor mutations in the other luxS mutant, CMPG5413, appear to compensate for the metabolic defects of the luxS mutation and to restore the resistance to gastric juice but cause a defect in adherence, biofilm formation, and exopolysaccharide production. The shorter residence time of this suppressor mutant in the murine gastrointestinal tract indicates a role for biofilm formation and exopolysaccharides in the persistence capacity of L. rhamnosus GG.
Bacteria of the genus Lactobacillus are investigated with growing interest in their health-promoting properties as common inhabitants of gut ecosystems or after consumption of marketed probiotic products. A probiotic bacterium is defined as “a live microorganism that, when administered in adequate amounts, confers a health benefit on the host” (14). The definition highlights that a probiotic bacterium needs to be viable at the target site to exert a health-promoting effect, with the main sites of action of probiotics being the small and large intestines. Therefore, an increasing amount of mechanistic studies are investigating how probiotic lactobacilli and bifidobacteria cope with various gastrointestinal tract (GIT)-related stress situations.
It was postulated that cell-cell communication plays an important role in various niches, such as the GIT, in coordinating bacterial behavior ranging from the maintenance of the commensal population to the induction of pathogenesis (19). LuxS has been widely investigated in relation to its role in bacterial cell-cell communication through the production of the signaling molecule autoinducer-2 (AI-2) (35). AI-2 is broadly produced by both gram-positive and gram-negative bacteria and seems to regulate niche-specific behavior such as bioluminescence in Vibrio harveyi (32) and biofilm formation of human commensal oral bacteria (26, 29). However, LuxS also forms an integral part of the activated methyl cycle (AMC), where it catalyzes the conversion of S-ribosylhomocysteine, yielding DPD [(S)-4,5-dihydroxy-2,3-pentanedione] and homocysteine (35). DPD undergoes spontaneous rearrangements to form AI-2, while homocysteine is recycled to methionine and S-adenosylmethionine (SAM). LuxS thus plays an important role in the central metabolism of SAM, which is used in various methylation reactions, and in the metabolism of the sulfur-containing amino acids methionine and cysteine, including a role in the biosynthesis of the important cellular antioxidant glutathione (39).
In this study, we investigated the in vivo role of LuxS in the human probiotic strain Lactobacillus rhamnosus GG. L. rhamnosus GG is currently one of the probiotics with the greatest amount of proven health benefits (13). Clinical trials have reported that L. rhamnosus GG can, among others, prevent and relieve certain types of diarrhea and atopic diseases and reduce inflammation in some inflammatory bowel diseases (13). Strain GG was isolated from a fecal sample of a healthy human adult based on its good tolerance to gastric acid and bile and its strong adherence capacity, among other criteria (16). We previously reported the effects of a luxS mutation on the in vitro growth and biofilm formation of L. rhamnosus GG (20, 21). Nutritional complementation experiments including cysteine supplementation could partially link the defects of the luxS mutant to the metabolic role of LuxS in the AMC. Moreover, we observed that a luxS mutation in L. rhamnosus GG can also induce unknown secondary suppressor mutations, pinpointing the complex role that LuxS appears to have in the physiology of L. rhamnosus GG (20). Here, we report the survival capacities of wild-type strain GG and two luxS mutants, a genuine luxS mutant, CMPG5412, and the putative suppressor mutant CMPG5413, for passage through the murine GIT. As the two luxS mutants behave very differently for GIT transit, we decided to phenotypically investigate the two luxS mutants in greater detail in relation to conditions and properties that could be relevant for residence in the GIT. Although we do not yet know the exact nature of the secondary mutation(s) in CMPG5413, the phenotype of this mutant with respect to biofilm formation and the production of extracellular polysaccharides is of interest for further study in relation to the probiotic properties of L. rhamnosus GG.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
The parental strains and plasmids that were used in this study are listed in Table 1. Escherichia coli cells were grown in Luria-Bertani broth (LB) medium with aeration at 37°C (28). L. rhamnosus GG and its derivatives were routinely grown at 37°C in de Man-Rogosa-Sharpe (MRS) medium (Difco) (12) under static conditions. Bacto Lactobacilli AOAC medium (Difco) and modified tryptic soy broth (mTSB) medium (21) were also used in this study. If required, antibiotics were applied at the following concentrations: 10 μg/ml tetracycline (Sigma), 100 μg/ml ampicillin (Sigma), 5 μg/ml (L. rhamnosus GG) or 100 μg/ml (E. coli) erythromycin (Fluka, Biochemika), and 50 μg/ml rifampin (Sigma).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or description | Reference and/or source |
|---|---|---|
| Strains | ||
| E. coli DH5α | F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK− mK−) supE44 λ−thi-1 girA96 relA1 | Gibco-BRL |
| L. rhamnosus GG | ||
| Wild type | Human isolate | ATCC 53103, 16 |
| LGG-Rifr | Rifr derivate of wild-type L. rhamnosus GG | This study |
| CMPG5340 | Wild-type L. rhamnosus GG derivative by insertion of pCMPG5340 at the tRNALeu locus; Eryr Tcr | This study |
| CMPG5412 | luxS knockout mutant of L. rhamnosus GG; luxS::tet(M); reduced growth capacity under N-limited conditions | 20 |
| CMPG5413 | luxS knockout mutant of L. rhamnosus GG; luxS::tet(M); putative suppressor mutant; wild-type growth capacity; no biofilm formation | 20 |
| CMPG5413-Rifr | Rifr derivate of strain CMPG5413 | This study |
| CMPG5413::pCMPG5339 | CMPG5413 derivative after introduction of a functional luxS gene on plasmid pCMPG5339; restored AI-2 production; no restored biofilm formation | This study |
| Plasmids | ||
| pCRII-TOPO | Cloning vector; Ampr Kanr | Invitrogen |
| pMD5057 | Plasmid from L. plantarum 5057 containing a tetracycline resistance marker, tet(M) | 10 |
| pEM40 | pUC19E-derived integration vector (attB located at the 3′ end of the tRNALeu locus) containing a 1.6-kb int-attP cassette of phage A2; Ampr Eryr | 2 |
| pCMPG5339 | pEM40-derived integration vector containing the luxSLGG gene with its putative P2 promoter | 20 |
| pCMPG5340 | pEM40-derived integration vector containing the Tcr marker from pMD5057 | This study |
DNA manipulations.
Routine molecular biology techniques were performed according to standard procedures (28). Restriction and modifying enzymes (from New England Biolabs) were used as recommended by the manufacturer. Plasmid DNA was prepared from Escherichia coli cells by use of Qiagen Miniprep kits. Chromosomal DNA and plasmid DNA were isolated from L. rhamnosus GG as previously described (11).
Construction of the Tcr-Eryr control strain CMPG5340.
The Tcr marker tet(M) was first amplified from Lactobacillus plantarum MD5057 (10) via PCR with Pro-221 (5′-GAATTCGAGATTCCTTTACAAATATGCTCTTAC-3′) and Pro-222 (5′-CGAATTCGTTCGGAATAGGTTATACTAGACAAAAG-3′). The Tcr marker was then blunt ligated into pEM40 (2) at the EcoRI site, resulting in plasmid pCMPG5340. This plasmid was transferred into wild-type strain GG, resulting in strain CMPG5340, by site-specific integration at the 3′ end of the tRNALeu locus, generating a functional hybrid gene (2, 11). Strain CMPG5340 was compared to wild-type strain GG for in vitro growth, biofilm formation, adherence to Caco-2 cells, and survival in simulated gastric juice as described below (data not shown), and no differences between the wild type and CMPG5340 were observed, indicating that CMPG5340 could be used as a control strain in the following mice experiments.
Determination of persistence capacity in murine GIT.
Groups of three 8-week-old female BALB/c mice were obtained from Harlan (Horst, The Netherlands) and housed in conventional filter-top cages. Food and water supply were given ad libitum. All experiments were performed under the approval of the Katholieke Universiteit Leuven Animal Experimentation Ethics Committee (project approval number P05045). The capacity of L. rhamnosus GG to survive passage through the murine gastrointestinal tract was determined by analysis of fecal samples by selective plating at different time points after oral administration. In order to distinguish L. rhamnosus GG from the endogenous murine lactobacilli, we used a Tcr-Eryr-marked strain (CMPG5340) or a rifampin-resistant (Rifr) derivative of wild-type strain GG, indicated in the text as the “wild-type control.” No phenotypic differences for these control strains in comparison with wild-type strain GG were observed for in vitro growth, biofilm formation, adherence to Caco-2 cells, and survival in simulated gastric juice (data not shown). We also confirmed that only exogenously applied L. rhamnosus GG bacteria from fecal samples could grow on MRS plates with 10 μg/ml tetracycline or 50 μg/ml rifampin (data not shown). The identity of L. rhamnosus GG was confirmed by colony morphology (large, creamy, white colonies on MRS agar) and a PCR with gene-specific primers for L. rhamnosus GG on randomly picked colonies with primers Pro-536 (5′-CAACACAAGCAAGGCGCTGA-3′) and Pro-537 (5′-GCGTCATTAATTTTAGCAGC-3′).
To compare the persistence capacities of wild-type control strains and luxS mutants, bacteria were grown for 24 h in MRS or AOAC medium. The next day, the optical density was measured at 600 nm, and estimated culture volumes to obtain the required cell number of the wild-type control and mutant were mixed after centrifugation, washing with phosphate-buffered saline (PBS), and resuspending at approximately 2 × 1010 CFU (mixed-strain suspension) per ml in PBS. The exact ratio of the wild-type control and mutants in the mixture was determined by plating a dilution series on selective medium. Groups of three mice received a 100-μl oral dose of the bacterial suspension (1:1 mixtures of mutant and wild-type control strains in a total dose of 2 × 109 CFU) by intragastric administration (gavage). Individual fecal samples were collected at different time points between 6 and 96 h postgavage. Fecal samples were weighed and subsequently homogenized and serially diluted in PBS. For some mice, tissue and content samples were collected from stomach, small bowel, cecum, and colon 48 h after gavage. Quantification of total L. rhamnosus GG populations in fecal or tissue samples was achieved by determining the CFU in triplicate for each sample on MRS agar plates containing 10 μg/ml Tc (for CMPG5430 and CMPG5412) or 50 μg/ml Rif (for LGG-Rifr and CMPG5413-Rifr). The percentage of mutant colonies was counted by replica plating of 200 colonies on MRS agar plates containing 5 μg/ml erythromycin (CMPG5412) or 10 μg/ml tetracycline (CMPG5413). Additionally, a control PCR was performed on randomly picked colonies to confirm their identity as wild-type control strain CMPG5340 versus the luxS mutant. Heretofore, we used the primer pairs Pro-564 (5′-AGCAGGACGAGAAAGCAATGAATGT-3′) and Pro-565 (5′-GCCGGTGTGGCGGAATTGGCAG-3′), specific for CMPG5340 (11), and Pro-240 (5′-AGGTAAGCTATTGAGAAGGAGATCC-3′) and Pro-249 (5′-GACATCTAATTATTTGTTCCCGCTATC-3′), specific for the integration of the tetracycline resistance marker (10), in the luxS gene. The ratios obtained after passage through the GIT were then compared to the actual ratio of the wild-type control and mutant before gavage (see above) and recalculated using a corresponding correction factor. All percentages given in the remainder of the text relate to these corrected values.
Survival in simulated gastric juice.
Simulated gastric juice was prepared and survival tests were performed as previously described (9). Simulated gastric juice was formulated using glucose (3.5 g/liter), NaCl (2.05 g/liter), KH2PO4 (0.60 g/liter), CaCl2 (0.11 g/liter), and KCl (0.37 g/liter), adjusted to pH 2.0 using 1 M HCl, and autoclaved at 121°C for 15 min. Porcine bile (Sigma) (0.05 g/liter), lysozyme (Sigma) (0.1 g/liter), and pepsin (Sigma) (13.3 mg/liter) were added as stock solutions prior to analysis. The percentages of survival were calculated by comparing the cell numbers before and after addition to simulated gastric juice at 0, 10, 30, 60, and 90 min. The experiment was performed in triplicate.
In vitro biofilm and adhesion assay.
The in vitro biofilm formation of wild-type GG and luxS mutants on polystyrene was assessed by crystal violet staining as previously described (21). Additionally, adhesion to the Caco-2 cell line was tested (24). The adhesion ratio (percentage) was calculated by comparing the cell number of the original bacterial suspension (109 CFU/ml) added to the final counts of the bacterial cell number after adhesion. All strains were tested in triplicate in three independent experiments.
EPS isolation and TEM analysis.
Cell-bound exopolysaccharide (EPS) (EPS-b) and EPS released into the culture medium (EPS-r) were isolated and quantified as previously described (21, 33). EPS-r was precipitated from culture supernatant with ethanol, while EPS-b was first extracted from the bacterial cells with 0.05 M EDTA prior to ethanol precipitation. For transmission electron microscopy (TEM) analysis, the bacteria were grown overnight in AOAC medium. Uncoated Cu grids (Stork Veco B.V., Eerbeek, The Netherlands) with a film of 7/1000 Pioloform (polyvinyl butyral) (Wacker) in chloroform (Hoechst) were used as a probe to adsorb bacterial cells. The grids were placed onto a drop of bacterial suspension for 15 s, incubated in 0.25% phosphotungstenic acid (pH 7) for 30 s, and washed three times, after which excess liquid was drained. The bacteria were observed with a Philips EM 208S transmission electron microscope at 80 kV.
RESULTS
L. rhamnosus GG survives passage through murine GIT.
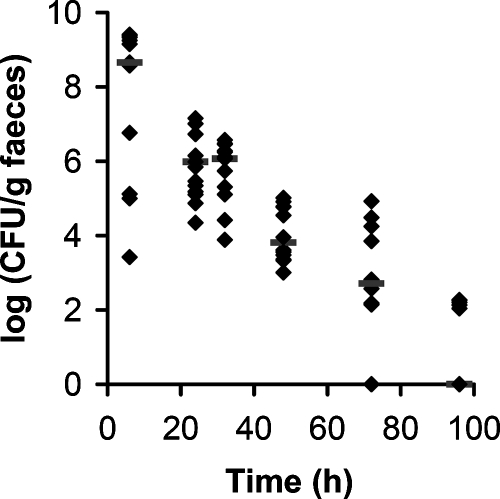
To investigate the persistence of L. rhamnosus GG in the murine GIT, we first investigated the recovery of L. rhamnosus GG (wild-type control CMPG5340) in murine feces at different time points after a single oral administration. L. rhamnosus GG has a typical colony morphology that facilitates isolation from fecal samples. Six hours after the gavage, L. rhamnosus GG started to appear in the feces of some, but not all, mice at concentrations of around 109 CFU/g feces (Fig. 1). Most of the administered L. rhamnosus GG cells were recovered in the feces between 6 h and 48 h after gavage (Fig. 1). After 96 h, only a few L. rhamnosus GG bacteria could still be retrieved. Although the transit time seemed to vary considerably among mice, the overall trend that most L. rhamnosus GG bacteria are shed into the feces between 6 and 48 h after administration was very similar. This gives an indication for the transit time of L. rhamnosus GG in mice after a single administration. The bacterial count of a fecal sample does not reflect events that might happen in the GIT, such as adherence, multiplication, or killing. However, knowing the number of L. rhamnosus GG bacteria retrieved in the feces and with an assumption of 100 to 200 mg feces per mouse per day, it seems that most of the administered L. rhamnosus GG bacteria survive GIT transit without considerable adherence. Moreover, given previous reports on the low rate of cell division by exogenous lactobacilli in the mouse gut (22), it can be speculated that the multiplication of L. rhamnosus GG in the murine GIT is low.
FIG. 1.
Population dynamics of L. rhamnosus GG in the murine GIT after a single gavage. Approximately 109 CFU of wild-type control strain CMPG5340 were administered to four groups of three conventionally raised mice, and the presence of L. rhamnosus GG in fecal pellets was monitored by selective plating at different time points after gavage. Values for individual mice are shown. Bars indicate medians.
luxS mutant strain CMPG5412 shows reduced survival within the murine GIT and simulated gastric juice.
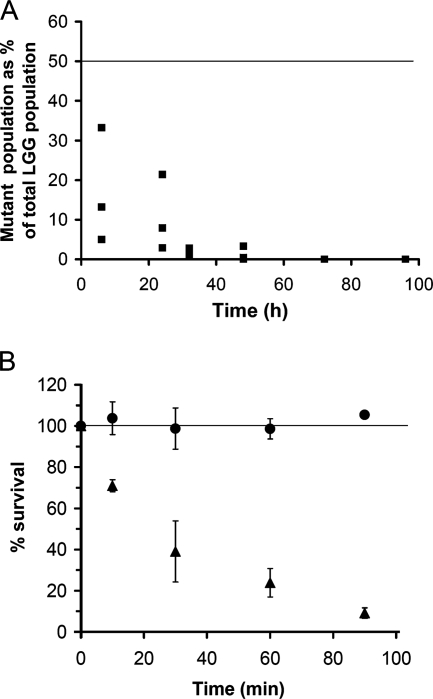
Having established that L. rhamnosus GG is able to survive passage through the murine GIT, we next investigated whether LuxS is required for this persistence capacity using a competition experiment. We previously showed that LuxS is required for the optimal in vitro growth of L. rhamnosus GG related to the role of LuxS in the AMC (20). luxS mutant strain CMPG5412 shows a growth defect under certain conditions such as cysteine and/or vitamin depletion (20). Comparison of the in vivo persistence percentages of the wild-type control (CMPG5340) and luxS mutant strain CMPG5412 (Fig. 2A) suggests that CMPG5412 is also severely affected in its capacity to persist within the GIT. While the initial percentage of the mutant was 50% of the total amount of bacterial cells administered to the mice (1:1 ratio with wild-type L. rhamnosus GG), this percentage rapidly declined after passage through the GIT to less than 1% at 48 h after gavage (Fig. 2A).
FIG. 2.
Characterization of the GIT survival capacity of luxS mutant strain CMPG5412. (A) Comparison of the wild-type control versus luxS mutant strain CMPG5412 for recovery in fecal samples. Mixtures of the wild-type control (CMPG5340) and mutant strain CMPG5412 (1:1) were administrated to three mice, and the percentage of mutants in the total L. rhamnosus GG (LGG) population, compared to an initial ratio of 50%, in fecal pellets was determined at different time points. Values for individual mice are shown. The line indicates the initial ratio of 50% mutant to 50% wild type (1:1) in the total L. rhamnosus GG population administered to the mice. (B) Comparison of wild-type control strain CMPG5340 versus luxS mutant strain CMPG5412 for survival in simulated gastric juice. The number of viable cells in simulated gastric juice was determined by plating a dilution series at different time points and expressed as a percentage of the initial numbers of cells added; circles indicate the wild type, and triangles indicate mutant strain CMPG5412. Data are the means of triplicate experiments, and error bars indicate standard deviations. The line indicates that the viable cell count at the initial time point was taken as 100%.
Since it is unlikely that there is considerable multiplication of L. rhamnosus GG in the murine GIT within the time frame of the experiment (see above), the main reason for the drastic shift in percentages between the wild type and luxS mutant strain CMPG5412 is not likely to be due solely to different growth rates of the wild type and the luxS mutant in the GIT, as one might speculate from our previous in vitro growth experiments (20). The in vivo data rather suggest enhanced killing of mutant strain CMPG5412 compared to the wild type in the GIT. The two main sources for bacterial killing in the GIT are gastric acidity and the action of bile (4). Therefore, we also investigated the stress resistance of mutant strain CMPG5412 to simulated gastric juice in vitro. As highlighted at the time of the isolation of L. rhamnosus GG (16) and confirmed in this study, wild-type L. rhamnosus GG shows very good resistance to gastric acidity and the action of gastric juice (Fig. 2B). In contrast, the concentration of viable cells of mutant strain CMPG5412 rapidly declined to less than 10% of the original population after 90 min of incubation in simulated gastric juice. Microscopic analysis with Live/Dead BacLight Bacterial Viability staining (Molecular Probes, Invitrogen) confirmed the results for the plate counts (data not shown). Therefore, LuxS seems to be required for optimal stress resistance against important GIT conditions such as gastric juice.
Suppressor mutant strain CMPG5413 rescued the capacity to survive within the GIT but shows less adherence.
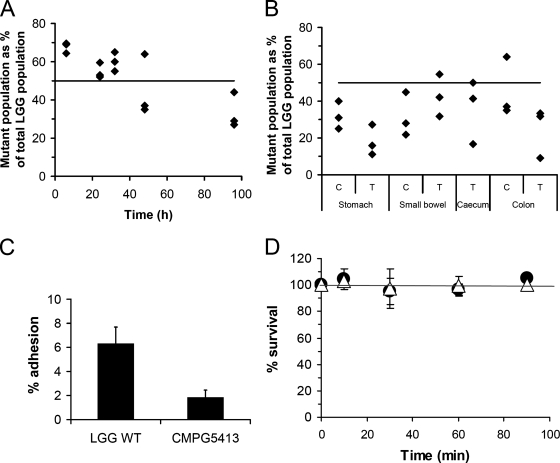
Together with luxS mutant strain CMPG5412, another luxS mutant (strain CMPG5413) was previously isolated (20). In contrast to CMPG5412, mutant strain CMPG5413 does not show a growth defect under conditions of cysteine and vitamin depletion (20). Several control experiments indicate that an unknown suppressor secondary-site mutation(s) must have occurred in CMPG5413, thereby compensating for the metabolic defects caused by the luxS mutation (20). Here, we wanted to investigate whether these unknown secondary mutations in CMPG5413 rescuing the in vitro growth defect could also restore the capacity to survive GIT transit. Comparing the persistence percentages of the wild-type control (LGG-Rifr) and mutant strain CMPG5413-Rifr indicated that CMPG5413 is indeed not attenuated in its ability to survive GIT transit (Fig. 3A). In fact, initial recovery (6 h postgavage) from fecal samples seems even higher for mutant strain CMPG5413 than for the wild-type control. As the percentage of the mutant thereafter gradually declines from ca. 70% to ca. 30% of the total L. rhamnosus GG population, it appears that CMPG5413 experiences a faster transit through the murine gut. Since preliminary experiments indicated a defect in adhesion and biofilm formation by CMPG5413 (see also below), tissue samples were also collected from three mice at 48 h postgavage. L. rhamnosus GG bacteria could be recovered from the tissue samples from the stomach, small intestine, cecum, and colon but at rather low numbers (ca. 102 to 103 CFU/g tissue). Comparison of the numbers of the wild type and the mutant suggests that of the small amount of exogenously applied L. rhamnosus GG cells that do adhere, more wild-type than mutant bacteria are adhering to tissue samples, especially on stomach and colon tissue (Fig. 3B). In addition, in vitro, we observed less adherence of CMPG5413 to Caco-2 cells (Fig. 3C). A lower recovery from the stomach could also indicate reduced acid tolerance. However, we did not observe significant differences between the wild type and CMPG5413 in an in vitro survival test in simulated gastric juice (Fig. 3D). Therefore, the observed lower recovery from the stomach at 48 h could indicate faster passage through the stomach and lower GIT, possibly because of reduced adherence and/or reduced biofilm formation (see below).
FIG. 3.
Characterization of the GIT survival capacity of suppressor mutant strain CMPG5413. (A) Comparison of the wild-type control versus CMPG5413 for recovery in fecal samples. Mixtures of the wild-type control (LGG-Rifr) and mutant strain CMPG5413-Rifr (1:1) were administrated to three mice, and the percentage of mutants in the total L. rhamnosus GG (LGG) population was determined in fecal pellets at different time points. Values for individual mice are shown. The line indicates the initial ratio of 50% mutant to 50% wild type (1:1) of the total L. rhamnosus GG population administered to the mice. (B) Comparison of wild-type control versus CMPG5413 for adhesion to different tissue samples. Tissue (T) and content (C) samples were collected from three mice 48 h after gavage. The percentage of mutants recovered is indicated, compared to an initial ratio of 50% of the total L. rhamnosus GG population (line) upon administration. Values for individual mice are shown. (C) Comparison of the wild type versus CMPG5413 for adhesion to Caco-2 cells. Wild-type (WT) L. rhamnosus GG and mutant strain CMPG5413 were grown overnight until stationary phase. The adherence percentage is a measure of the ratio of the number of bacteria after adhesion to the initial number of bacteria added to the Caco-2 cells (ca. 109 CFU/ml). Each experiment was repeated at least three times, and error bars indicate standard deviations. (D) Comparison of the wild type versus CMPG5413 for survival in simulated gastric juice. The number of viable cells in simulated gastric juice was determined by plating a dilution series at different time points and expressed as a percentage of the initial numbers of cells added; circles indicate the wild type, and triangles indicate mutant strain CMPG5413. Data are the means of triplicate experiments, and error bars indicate standard deviations.
CMPG5413 is strongly attenuated in in vitro biofilm formation due to reduced EPS production.
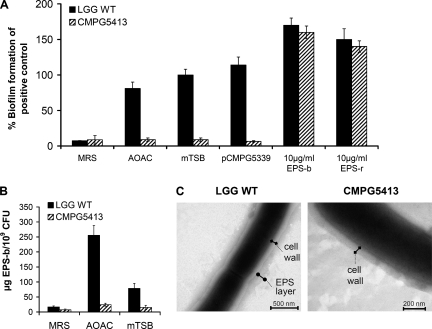
Finally, we wanted to further characterize the reduced adherence capacity of CMPG5413. Besides reduced adherence to GIT tissue and cultured Caco-2 cells, CMPG5413 is unable to form biofilms under all conditions tested (Fig. 4A). Since this biofilm defect cannot be complemented genetically with a functional copy of the luxS gene (Fig. 4A) or nutritionally, in contrast to CMPG5412 (20, 21), it can be inferred that CMPG5413 shows a structural biofilm defect as a consequence of a secondary mutation(s). Therefore, we decided to investigate this phenotype in greater detail, especially because the biofilm defect is very prominent and might have implications for further in vivo studies. Extracellular polysaccharides are one of the main components of a biofilm matrix (5). When we isolated and quantified EPS production of the wild type and strain CMPG5413, CMPG5413 produced significantly less EPS-b (Fig. 4B). The greatest reduction was observed in AOAC medium, where CMPG5413 showed about 1/10 of the EPS-b amount of the wild-type. Also, a reduction in EPS-r of CMPG5413 could be observed in AOAC medium (data not shown). TEM analysis confirmed the reduced amount of EPS-b in CMPG5413. After growth in AOAC medium, wild-type L. rhamnosus GG appeared to have a hairy EPS layer around the cell wall, which was almost absent in CMPG5413 (Fig. 4C). Atomic force microscopy and single-molecule force spectroscopy also revealed a significant reduction in the distribution and length of the cell surface polysaccharides of CMPG5413 compared to those of the wild type (unpublished results). To confirm that the unknown spontaneous mutation affected EPS production in CMPG5413 and, in this way, biofilm formation, we attempted to complement the biofilm formation of CMPG5413 by the addition of exogenous EPS isolated from the wild type (Fig. 4A). At a final concentration of around 10 μg/ml EPS, complementation could be achieved. The addition of EPS even increased the biofilm formation of both the wild type and mutant, showing that EPS is an important factor in biofilm formation by L. rhamnosus GG.
FIG. 4.
Characterization of the biofilm defect of CMPG5413. (A) Biofilm formation under different conditions. Biofilm formation of wild-type L. rhamnosus GG (LGG WT) in mTSB medium was included as a positive control and set to 100%. Complementation of biofilm formation by CMPG5413 was attempted by genetic complementation with a functional luxS gene on plasmid pCMPG5339 and by the addition of 10 μg/ml EPS to mTSB medium. (B) Comparison of EPS-b production in different media. Data are the means of triplicate experiments, and error bars indicate standard deviations. (C) TEM analysis of wild-type L. rhamnosus GG and mutant strain CMPG5413. The cell wall and EPS layer (absent in mutant) are indicated.
DISCUSSION
To our knowledge, this is the first report on the effect of a mutation on the survival and persistence capacity of the human “prototypical” probiotic strain L. rhamnosus GG within the GIT of conventionally raised mice. After a single oral administration, most L. rhamnosus GG cells seem to pass into the feces between 6 h and 48 h after the gavage, an indication of the transit time after a single administration. L. rhamnosus GG was previously selected based on its good capacity of resistance to acid and bile in vitro (16) and was previously shown to survive passage through the human GIT (1). Based on the number of L. rhamnosus GG bacteria retrieved in the murine feces and with an assumption of 100 to 200 mg feces per mouse per day, we can estimate that most of the administrated wild-type L. rhamnosus GG also survives within the murine GIT. Given the fact that we observed clear differences between the wild type and mutant strain CMPG5412, conventionally raised mice provide a good model system to study the survival capacity of a probiotic strain within the GIT. Moreover, this survival capacity seems to correlate well with the capacity to survive gastric stress, as can be estimated from in vitro experiments with simulated gastric juice, although other factors might play an important role in GIT survival.
Our data indicate that L. rhamnosus GG does not seem to establish itself for a long time in significant numbers in the murine GIT after a single administration. However, analysis of fecal samples gives only a general idea of the number of bacteria that transit through the GIT. It is likely that a small amount of the administered bacteria is able to adhere and multiply in certain microhabitats, albeit at a low rate, as described previously for Lactobacillus casei (22) and as also observed in this study, given the rather low numbers of L. rhamnosus GG bacteria that could be recovered from the tissue samples 48 h after a single gavage. Nevertheless, conventionally raised mice have a rather stable GIT microbiota that, according to the niche exclusion and colonization resistance principle, is quite resistant to colonization with new lactobacilli under normal healthy conditions, especially after a single administration. Therefore, probiotics can only temporarily colonize the new host, so most researchers agree that probiotics need to be taken daily to obtain health-promoting effects (4). Germ-free mice might be a more suitable model system to investigate the in vivo molecular mechanisms of adhesion to gastrointestinal tissues and colonization capacity. This is further supported by the fact that L. rhamnosus GG was previously shown to more permanently colonize the digestive tract of germ-free mice after a single administration (18). In these germ-free mice, L. rhamnosus GG was found to be associated with the mucosa of both the stomach and the intestine, and this association increased from the proximal to the distal intestine (18).
As to the specific impact of the luxS mutation on the in vivo persistence of L. rhamnosus GG, we observed that luxS mutant strain CMPG5412 was severely affected in its capacity to survive GIT transit. The main cause for this reduced ecological fitness seems to be enhanced killing in the GIT, especially in the stomach, as confirmed by in vitro experiments with simulated gastric juice. Moreover, the in vivo nutritional environment of the GIT was apparently not able to extracellularly complement the defect of a luxS mutation and prevent killing in time. However, the direct role of LuxS and the AMC in stress resistance is not completely understood. Glutathione, synthesized from cysteine and thus directly coupled to the AMC, is an important biomarker for oxidative stress and might play an important role in in vivo survival. In addition to its key role in maintaining the proper oxidation state of protein thiol groups, glutathione also serves a key function in protecting the bacterial cell from the action of low pH, chlorine compounds, and osmotic stresses (23). Additionally, mutations in genes encoding enzymes of the AMC could also influence stress resistance by affecting polyamine synthesis or various SAM-dependent methylation reactions (39).
Several studies indeed support a role for LuxS, the AMC, and/or glutathione in (GIT-related) stress resistance. In vitro, LuxS was previously related to acid resistance in different bacteria such as Lactobacillus acidophilus (3), Lactococcus lactis (15), Streptococcus mutans (38), and E. coli (31). In addition to LuxS, genome-wide screens in L. plantarum have identified glutathione reductase, cystathionine beta-lyase, and cysteine synthase (metC-cysK operon) as being induced upon bile treatment (7, 8). Intriguingly, in vivo expression technology (IVET) studies investigating the genetic background of the survival and persistence capacity of Lactobacillus reuteri 100-23 (36) and L. plantarum (6) in mice identified a common gene encoding a conserved hypothetical protein in both lactobacilli. This conserved protein shows homology with a putative vitamin B12-independent methionine synthase, also referred to as YxjH (17, 27). YxjH is suggested to be an alternative methionine synthase involved in SAM recycling and the AMC (27), but this is not yet experimentally validated. Nevertheless, the identification of this gene in these IVET screens indicates that the AMC is important in vivo in lactobacilli. In addition, several IVET screens in other bacteria have revealed genes playing a role in glutathione synthesis or glutathione reduction that are induced during interactions with animal hosts (reviewed in reference 25). Finally, mutation of the luxS gene was shown to affect the ecological performance of the murine commensal L. reuteri strain 100-23 in ex-Lactobacillus-free mice (34), confirming its important in vivo function in lactobacilli.
Besides CMPG5412, we previously isolated a putative suppressor luxS mutant strain, CMPG5413 (20), which shows a totally different phenotypic pattern. In strain CMPG5413, the metabolic defects of a luxS mutation seem to be rescued by an unknown secondary mutation(s), as evidenced by its wild-type growth capacity under all conditions tested (20) and the fact that we could not genetically complement in vitro biofilm formation. The occurrence of suppressor mutations further highlights the physiologically important role of LuxS. Here, we report that the putative suppressor mutant CMPG5413 has also rescued its capacity to survive passage through the GIT. However, this rescue seems to be at the expense of the adhesion capacity of CMPG5413. CMPG5413 shows a tendency toward reduced adhesion to gastrointestinal tissues and cultured Caco-2 cells but has especially lost its in vitro biofilm formation capacity under all conditions tested. It would be interesting to identify this secondary mutation(s), especially since the occurrence of second-site mutations in luxS mutant strains that abolish biofilm formation might also occur in other bacteria, as previously suggested (35). We could already indirectly demonstrate that this suppressor mutation(s) affects EPS production in CMPG5413, as it produces less EPS, and the biofilm formation of CMPG5413 could be restored by the exogenous addition of EPS.
Since CMPG5413 shows a structural biofilm defect and reduced EPS production, studying its in vivo performance could give indications on the in vivo importance of these phenotypes. Although its survival capacity is not affected, CMPG5413 seems to experience a shorter residence time in the GIT, albeit not greatly reduced, in comparison to that of wild-type strain GG. This faster transit might be related to the structural biofilm defect of CMPG5413. Sonnenburg et al. (30) previously proposed that biofilm formation in food particles, the mucus layer, and/or the mucosa increases the persistence capacity of bacteria in the GIT. Additionally, EPS might be crucial for this in vivo biofilm formation while being less important for acid resistance; this is in contrast to the major stress resistance role of LuxS as elaborated above. EPS molecules have already been hypothesized to increase the residence time of lactic acid bacteria in the gastrointestinal tract (37). Clearly, the role of EPS and biofilm formation in the persistence capacity and probiotic action of L. rhamnosus GG is of great interest for future investigations.
Acknowledgments
S.L. and S.C.J.D.K. are research assistants of the FWO-Vlaanderen. I.J.J.C. holds a grant from the IWT-Vlaanderen. This work was partially supported by the FWO-Vlaanderen.
We gratefully acknowledge P. Augustijns for providing the Caco-2 cells used in this study and M. Alvarez and M. Danielsen for pEM40 and pMD5057. Additionally, we thank our group members who helped with the replica plating.
Footnotes
Published ahead of print on 6 June 2008.
REFERENCES
- 1.Alander, M., R. Satokari, R. Korpela, M. Saxelin, T. Vilpponen-Salmela, T. Mattila-Sandholm, and A. von Wright. 1999. Persistence of colonization of human colonic mucosa by a probiotic strain, Lactobacillus rhamnosus GG, after oral consumption. Appl. Environ. Microbiol. 65:351-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez, M. A., M. Herrero, and J. E. Suarez. 1998. The site-specific recombination system of the Lactobacillus species bacteriophage A2 integrates in gram-positive and gram-negative bacteria. Virology 250:185-193. [DOI] [PubMed] [Google Scholar]
- 3.Azcarate-Peril, M. A., O. McAuliffe, E. Altermann, S. Lick, W. M. Russell, and T. R. Klaenhammer. 2005. Microarray analysis of a two-component regulatory system involved in acid resistance and proteolytic activity in Lactobacillus acidophilus. Appl. Environ. Microbiol. 71:5794-5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bezkorovainy, A. 2001. Probiotics: determinants of survival and growth in the gut. Am. J. Clin. Nutr. 73:399S-405S. [DOI] [PubMed] [Google Scholar]
- 5.Branda, S. S., S. Vik, L. Friedman, and R. Kolter. 2005. Biofilms: the matrix revisited. Trends Microbiol. 13:20-26. [DOI] [PubMed] [Google Scholar]
- 6.Bron, P. A., C. Grangette, A. Mercenier, W. M. de Vos, and M. Kleerebezem. 2004. Identification of Lactobacillus plantarum genes that are induced in the gastrointestinal tract of mice. J. Bacteriol. 186:5721-5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bron, P. A., M. Marco, S. M. Hoffer, E. Van Mullekom, W. M. de Vos, and M. Kleerebezem. 2004. Genetic characterization of the bile salt response in Lactobacillus plantarum and analysis of responsive promoters in vitro and in situ in the gastrointestinal tract. J. Bacteriol. 186:7829-7835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bron, P. A., D. Molenaar, W. M. de Vos, and M. Kleerebezem. 2006. DNA micro-array-based identification of bile-responsive genes in Lactobacillus plantarum. J. Appl. Microbiol. 100:728-738. [DOI] [PubMed] [Google Scholar]
- 9.Corcoran, B. M., C. Stanton, G. F. Fitzgerald, and R. P. Ross. 2005. Survival of probiotic lactobacilli in acidic environments is enhanced in the presence of metabolizable sugars. Appl. Environ. Microbiol. 71:3060-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danielsen, M. 2002. Characterization of the tetracycline resistance plasmid pMD5057 from Lactobacillus plantarum 5057 reveals a composite structure. Plasmid 48:98-103. [DOI] [PubMed] [Google Scholar]
- 11.De Keersmaecker, S. C., K. Braeken, T. L. Verhoeven, M. Perea Vélez, S. Lebeer, J. Vanderleyden, and P. Hols. 2006. Flow cytometric testing of green fluorescent protein-tagged Lactobacillus rhamnosus GG for response to defensins. Appl. Environ. Microbiol. 72:4923-4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Man, J. C., M. Rogosa, and M. E. Sharpe. 1960. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 23:130-135. [Google Scholar]
- 13.Doron, S., D. R. Snydman, and S. L. Gorbach. 2005. Lactobacillus GG: bacteriology and clinical applications. Gastroenterol. Clin. N. Am. 34:483-498. [DOI] [PubMed] [Google Scholar]
- 14.FAO/WHO. 2001. Report on joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. WHO, Geneva, Switzerland.
- 15.Frees, D., F. K. Vogensen, and H. Ingmer. 2003. Identification of proteins induced at low pH in Lactococcus lactis. Int. J. Food Microbiol. 87:293-300. [DOI] [PubMed] [Google Scholar]
- 16.Gorbach, S. L. 1996. The discovery of Lactobacillus GG. Nutr. Today 31:2S-4S. [Google Scholar]
- 17.Grundy, F. J., and T. M. Henkin. 1998. The S box regulon: a new global transcription termination control system for methionine and cysteine biosynthesis genes in gram-positive bacteria. Mol. Microbiol. 30:737-749. [DOI] [PubMed] [Google Scholar]
- 18.Hudault, S., V. Lievin, M. F. Bernet-Camard, and A. L. Servin. 1997. Antagonistic activity exerted in vitro and in vivo by Lactobacillus casei (strain GG) against Salmonella typhimurium C5 infection. Appl. Environ. Microbiol. 63:513-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaper, J. B., and V. Sperandio. 2005. Bacterial cell-to-cell signaling in the gastrointestinal tract. Infect. Immun. 73:3197-3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lebeer, S., S. C. De Keersmaecker, T. L. Verhoeven, A. A. Fadda, K. Marchal, and J. Vanderleyden. 2007. Functional analysis of luxS in the probiotic strain Lactobacillus rhamnosus GG reveals a central metabolic role important for growth and biofilm formation. J. Bacteriol. 189:860-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lebeer, S., T. L. A. Verhoeven, M. Perea Vélez, J. Vanderleyden, and S. C. J. De Keersmaecker. 2007. Impact of environmental and genetic factors on biofilm formation by the probiotic strain Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 73:6768-6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee, Y. K., P. S. Ho, C. S. Low, H. Arvilommi, and S. Salminen. 2004. Permanent colonization by Lactobacillus casei is hindered by the low rate of cell division in mouse gut. Appl. Environ. Microbiol. 70:670-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masip, L., K. Veeravalli, and G. Georgioui. 2006. The many faces of glutathione in bacteria. Antioxid. Redox Signal. 8:753-762. [DOI] [PubMed] [Google Scholar]
- 24.Perea Vélez, M., T. Verhoeven, C. Draing, S. Von Aulock, M. Pfitzenmaier, A. Geyer, I. Lambrichts, C. Grangette, B. Pot, J. Vanderleyden, and S. C. J. De Keersmaecker. 2007. Functional analysis of d-alanylation of lipoteichoic acid in the probiotic strain Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 73:3595-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rediers, H., P. B. Rainey, J. Vanderleyden, and R. De Mot. 2005. Unraveling the secret lives of bacteria: use of in vivo expression technology and differential fluorescence induction promoter traps as tools for exploring niche-specific gene expression. Microbiol. Mol. Biol. Rev. 69:217-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rickard, A. H., R. J. Palmer, Jr., D. S. Blehert, S. R. Campagna, M. F. Semmelhack, P. G. Egland, B. L. Bassler, and P. E. Kolenbrander. 2006. Autoinducer 2: a concentration-dependent signal for mutualistic bacterial biofilm growth. Mol. Microbiol. 60:1446-1456. [DOI] [PubMed] [Google Scholar]
- 27.Rodionov, D. A., A. G. Vitreschak, A. A. Mironov, and M. S. Gelfand. 2004. Comparative genomics of the methionine metabolism in gram-positive bacteria: a variety of regulatory systems. Nucleic Acids Res. 32:3340-3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 29.Shao, H., R. J. Lamont, and D. R. Demuth. 2007. Autoinducer 2 is required for biofilm growth of Aggregatibacter (Actinobacillus) actinomycetemcomitans. Infect. Immun. 75:4211-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonnenburg, J. L., L. T. Angenent, and J. I. Gordon. 2004. Getting a grip on things: how do communities of bacterial symbionts become established in our intestine? Nat. Immunol. 5:569-573. [DOI] [PubMed] [Google Scholar]
- 31.Stancik, L. M., D. M. Stancik, B. Schmidt, D. M. Barnhart, Y. N. Yoncheva, and J. L. Slonczewski. 2002. pH-dependent expression of periplasmic proteins and amino acid catabolism in Escherichia coli. J. Bacteriol. 184:4246-4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Surette, M. G., M. B. Miller, and B. L. Bassler. 1999. Quorum sensing in Escherichia coli, Salmonella typhimurium and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc. Natl. Acad. Sci. USA 96:1639-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tallon, R., P. Bressollier, and M. C. Urdaci. 2003. Isolation and characterization of two exopolysaccharides produced by Lactobacillus plantarum EP56. Res. Microbiol. 154:705-712. [DOI] [PubMed] [Google Scholar]
- 34.Tannock, G. W., S. Ghazally, J. Walter, D. Loach, H. Brooks, G. Cook, M. Surette, C. Simmers, P. Bremer, F. Dal Bello, and C. Hertel. 2005. Ecological behavior of Lactobacillus reuteri 100-23 is affected by mutation of the luxS gene. Appl. Environ. Microbiol. 71:8419-8425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vendeville, A., K. Winzer, K. Heurlier, C. M. Tang, and K. R. Hardie. 2005. Making ‘sense’ of metabolism: autoinducer-2, LuxS and pathogenic bacteria. Nat. Rev. Microbiol. 3:383-396. [DOI] [PubMed] [Google Scholar]
- 36.Walter, J., N. C. K. Heng, W. P. Hammes, D. M. Loach, G. W. Tannock, and C. Hertel. 2003. Identification of Lactobacillus reuteri genes specifically induced in the mouse gastrointestinal tract. Appl. Environ. Microbiol. 69:2044-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welman, A. D., and I. S. Maddox. 2003. Exopolysaccharides from lactic acid bacteria: perspectives and challenges. Trends Biotechnol. 21:269-274. [DOI] [PubMed] [Google Scholar]
- 38.Wen, Z. T., and R. A. Burne. 2004. LuxS-mediated signaling in Streptococcus mutans is involved in regulation of acid and oxidative stress tolerance and biofilm formation. J. Bacteriol. 186:2682-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winzer, K., K. R. Hardie, and P. Williams. 2003. LuxS and autoinducer-2: their contribution to quorum sensing and metabolism in bacteria. Adv. Appl. Microbiol. 53:291-396. [DOI] [PubMed] [Google Scholar]