Abstract
The clinical presentation of meningococcal disease is closely related to the number of meningococci in the circulation. This study aimed to examine the activation of the innate immune system after being exposed to increasing and clinically relevant concentrations of meningococci. We incubated representative Neisseria meningitidis serogroup B (ST-32) and serogroup C (ST-11) strains and a lipopolysaccharide (LPS)-deficient mutant (the 44/76 lpxA mutant) in human serum and whole blood and measured complement activation and cytokine secretion and the effect of blocking these systems. HEK293 cells transfected with Toll-like receptors (TLRs) were examined for activation of NF-κB. The threshold for cytokine secretion and activation of NF-κB was 103 to 104 meningococci/ml. LPS was the sole inflammation-inducing molecule at concentrations up to 105 to 106 meningococci/ml. The activation was dependent on TLR4-MD2-CD14. Complement contributed to the inflammatory response at ≥105 to 106 meningococci/ml, and complement activation increased exponentially at ≥107 bacteria/ml. Non-LPS components initiated TLR2-mediated activation at ≥107 bacteria/ml. As the bacterial concentration exceeded 107/ml, TLR4 and TLR2 were increasingly activated, independent of CD14. In this model mimicking human disease, the inflammatory response to N. meningitidis was closely associated with the bacterial concentration. Therapeutically, CD14 inhibition alone was most efficient at a low bacterial concentration, whereas addition of a complement inhibitor may be beneficial when the bacterial load increases.
Neisseria meningitidis is still a much-feared pathogen owing to its propensity to cause epidemic meningitis and fulminant septicemia (34, 35). After breaching the mucosal barrier in the upper respiratory tract, invasive meningococci enter the circulation and start to proliferate. The growth velocity in the vasculature is a major determinant of the clinical presentation. A majority of patients reveal a comparatively low-grade meningococcemia, which subsequently seeds the subarachnoid space, where meningococci proliferate rapidly, leading to meningitis without shock symptoms. A minority of patients develop fulminant meningococcal septicemia, characterized by very rapid bacterial growth in the circulation (3, 7, 34, 35). The massive bacterial growth is reflected in high levels of endotoxin (lipopolysaccharide [LPS]), complement activation products, and cytokines in plasma (5, 6, 13, 23, 36, 37). The real bacterial loads in patients with fulminant meningococcemia have been quantified to be 105 to 108 bacteria/ml by determining the numbers of N. meningitidis DNA molecules, using robotized magnetic bead extraction of the bacterial DNA and real-time PCR (12, 25).
The complement system is of special interest with regard to meningococcal disease. Activation of complement leads to deposition of the opsonic factor C3b/iC3b, with subsequent phagocytosis and intracellular killing of bacteria and insertion of the C5b-9 complex in the bacterial membrane, with subsequent killing of the bacteria by lysis. Certain deficiencies of the complement system, in particular those in properdin and the terminal C5-C9 components, are associated with a higher risk for meningococcal disease (21). On the other hand, it has been documented that massively increased systemic complement activation is correlated with a fatal outcome of meningococcal sepsis (6, 14), and there is growing experimental evidence indicating that complement may play a disadvantageous role in sepsis, principally by C5a-mediated effects (11, 39).
LPS, the major inflammatory constituent in the outer membranes of meningococci, acts as a ligand to the Toll-like receptor 4 (TLR4)-MD2 complex (8, 28). Other components in the outer membrane, including lipoproteins and porins, have been shown to provoke inflammatory reactions by binding to TLR2 (15, 16, 20, 26). Activation of TLR2 and TLR4 is facilitated by CD14 (1). Other TLRs, including TLR9, which is activated by CpG DNA, could potentially also be involved in the inflammatory response to meningococci (22). Binding of ligands to TLRs leads to activation of the key transcription factor NF-κB, which controls the expression of an array of inflammatory cytokine genes (18).
There is a need for a more precise insight into the interactions between the different inflammatory signaling systems at different stages of meningococcemia. The aim of this study was to examine to what extent innate immunity is activated by meningococci at increasing concentrations corresponding to those found in different clinical presentations of meningococcal disease. For this purpose, we studied the relative contributions and interaction of two main branches of the innate immune system, namely, complement and the TLR system. To elucidate the specific role of LPS in the inflammatory reaction, we examined the effects of two wild-type meningococcal strains representing major pathogenic clones, i.e., multilocus sequence type 32 (ST-32) and ST-11, with LPS integrated in the outer membrane, and compared the results with those for an LPS-deficient mutant strain (the 44/76 lpxA mutant) derived from the ST-32 serogroup B 44/76 strain. By selectively blocking CD14 and complement, the individual and collective contributions of each of these systems to cytokine production were investigated, and by using various TLR-transfected cell lines, the roles of individual TLRs were studied. The data indicate a complex and variable interaction between the two parts of innate immunity, with different effects depending on the dose of bacteria.
MATERIALS AND METHODS
Equipment and reagents.
All materials used in the experiments where cytokines or NF-κB activity was measured were endotoxin free. The polypropylene tubes used were Nunc cryotubes (Nalgene Nunc, Roskilde, Denmark). Lepirudin (Refludan) was purchased from Hoechst (Frankfurt am Main, Germany). Dulbecco's phosphate-buffered saline (PBS) from Invitrogen Corp. (Carlsbad, CA) was used as a buffer solution.
Monoclonal antibodies.
The monoclonal mouse anti-human CD14 immunoglobulin G1 antibody 60bca (ATCC HB-247) was used for inhibition of CD14. Monoclonal mouse anti-human immunoglobulin G1 antibody (1B9; Diatec Monoclonal Antibodies AS, Oslo, Norway) was used as an isotype control. The optimal anti-CD14 concentration for cytokine inhibition was found by preliminary studies to be 50 μg/ml.
Complement inhibition.
Complement activation was blocked at the C3 level by compstatin {Ac-I[CV(1MeW)QDWGAHRC]T}, a 13-amino-acid cyclic peptide that binds to and inhibits cleavage of C3 (17). The concentration used (25 μM) was shown to completely block the formation of the terminal C5b-to-C9 complement complex (TCC). Furthermore, the effect of C5a was blocked by the synthetic cyclic hexapeptide AcF[OPdChaWR], previously characterized as an efficient C5a receptor (C5aR) antagonist (9).
Complement activation assays.
The soluble TCC was measured in an enzyme immunoassay, principally as described originally (24). The assay is based on a monoclonal antibody (aE11) recognizing a neoepitope exposed in C9 after it has been inserted in the TCC.
Quantification of cytokines.
Tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), IL-6, and IL-8 were measured on a Bioplex assay reader (Luminex 100; Bio-Rad Laboratories, Hercules, CA), using a Bio-Plex human cytokine panel (Bio-Rad Laboratories).
Bacterial strains.
The international reference strain N. meningitidis 44/76 (also denoted H44/76) is characterized as B:15:P1.7,16:L3,7,9. It is a representative strain belonging to the ST-32 clone. The N. meningitidis 44/76 lpxA mutant (also denoted the H44/76 lpxA mutant) is a viable encapsulated isogenic mutant of strain 44/76 that completely lacks LPS in the outer membrane. It was constructed by insertional inactivation of the lpxA gene, which is essential for the first committed step of biosynthesis of LPS, as described by Steeghs et al. (33). For the present study, batch suspensions of the 44/76 lpxA mutant were tested and showed no reactivity in a highly specific and sensitive LPS test, the Limulus amebocyte lysate assay (Pyrochrome, Associated of Cape Cod Inc., MA). The expression level of the integral outer membrane proteins by the LPS-deficient mutant is slightly higher than that by the wild-type strain. The outer membrane phospholipid composition is altered, with a switch to mostly short-chain, saturated fatty acids (32). N. meningitidis 151/85 (from the strain collection of the National Institute of Public Health, Oslo, Norway) is characterized as C:2a:P1.2:L3,9. It is a representative strain of the ST-11 clone and was isolated from a 6-month-old boy who died from fulminant meningococcal septicemia in 1985. Pathogenic meningococci belonging to the ST-32 and ST-11 clones have been isolated all over the world. Meningococci were grown overnight on Colombia agar and resuspended in sterile PBS. Bacteria were heat inactivated at 56°C for 30 min and then frozen at −70°C until they were used.
Purified N. meningitidis LPS.
LPS was extracted from strain 44/76 as described previously (4), and the biological strength of 100 pg/ml was determined to be 3.3 endotoxin units (EU)/ml by Limulus amebocyte lysate assay. The final product contained <0.3% protein and no particular nucleic acid (4).
Determination of number of meningococci.
Quantification of the number of N. meningitidis DNA copies was performed by robotized bead extraction of bacterial DNA and real-time PCR (LightCycler; Roche Diagnostics GmbH, Mannheim, Germany), as described previously (25).
Complement activation in serum.
Sera from 10 healthy donors were activated by twofold increasing concentrations of 44/76 at 37°C for 30 min. Similarly, sera from three donors were activated by 151/85 and the 44/76 LPS-deficient mutant at equal concentrations. The range of bacterial concentrations used was based on preliminary studies with 10-fold serial dilutions. After activation, the samples were placed on ice and EDTA was added to stop further complement activation. The sera were frozen at −70°C until analysis for complement (TCC) activation.
Whole-blood experiments.
Lepirudin-anticoagulated whole blood from healthy donors was preincubated shortly after it was withdrawn with inhibitors or negative control reagents in a 37°C water bath for 20 min before adding meningococci simultaneously at 10-fold increasing concentrations. The blood samples were then incubated on rollers for 2 hours in a 37°C climate-controlled room. Thereafter, the samples were placed on ice, and EDTA was added before centrifugation. The plasma supernatants were collected and frozen at −70°C for later analysis of cytokines and complement activation. Blood samples from five donors were included in the experiments comparing cytokine secretion by 44/76 and the 44/76 LPS-deficient mutant. The same number of donors was included in the inhibitor studies, except for those with 44/76 at 105 to 107 bacteria/ml, where samples from eight donors were included. Samples from three donors were included in the experiments with 151/85.
Transient transfection and luciferase assay.
Human embryonic kidney 293 (HEK293) cells (ATCC, Manassas, VA) were grown in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal calf serum (Euroclone, Milano, Italy), l-glutamine, and 10 μg/ml ciprofloxacin (Cellgro, Manassas, VA) at 37°C and 8% CO2. Transient transfection was done using GeneJuice transfection reagent (Novagen, Merck KGaA, Darmstadt, Germany) according to the manufacturer's protocol. In short, cells were plated at a cell density of 1 × 104 cells/well in 96-well dishes and grown to 50% confluence. Plasmids used were the NF-κB-dependent luciferase reporter plasmid pELAM-luc (8); pcDNA3 expressing human CD14, kindly provided by D. Golenbock (University of Massachusetts Medical School); pEFBOS expressing human MD2, kindly provided by K. Miyake (University of Tokyo); pcDNA3 expressing human TLR4, kindly provided by R. Medzhitov and C. Janeway (Yale University, New Haven, CT); and pRK7 expressing human TLR2, kindly provided by C. Kirschning (Technical University of Munich). Each plasmid was transfected at a dosage of 25 ng/well, and pcDNA3 (Invitrogen) was used to adjust the total amount of plasmid to 100 ng/well. All plasmids were isolated using an EndoFree plasmid kit (Qiagen Inc., Valencia, CA). After incubation for 24 h, the cells were stimulated for 18 h with 44/76, the 44/76 LPS-deficient mutant, and purified N. meningitidis LPS at 10-fold increasing concentrations. HEK293 cells stably transfected with TLR9 were generously provided by Eisai Pharmaceuticals (Andover, MA). The positive controls were stimulated with 10 μM phosphothioate CpG DNA 2006 (Tib Molbiol, Berlin, Germany). Cytoplasmic extracts were prepared, and luciferase activity was measured using a luciferase assay system kit according to the manufacturer's recommendations (Promega, Madison, WI), using a Victor 1420 multilabel counter (Perkin Elmer, Waltham, MA). Results for triplicate wells (duplicate wells for the TLR9-transfected cells) are given as levels of induction relative to the PBS-treated control.
Statistics.
Statistical analyses were performed with GraphPad Prism for Windows, version 4.00, from GraphPad Software (San Diego, CA). One-way or two-way analysis of variance (ANOVA) was used, as specified in the legends. If the overall P value was <0.05, considered the level of significance, Bonferroni posttest analysis was performed to compare individual groups in the complement activation experiments and the inhibitor studies.
RESULTS
Complement activation by 44/76, 151/85, and the 44/76 LPS-deficient mutant, as measured by the TCC level.
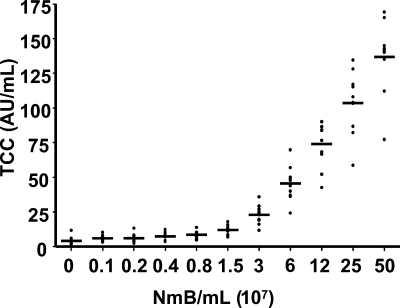
The concentration of TCC was similar to the background level at ≤106 bacteria/ml serum. Higher bacterial concentrations induced a dose-dependent increase, reaching a mean value of 140 arbitrary units/ml at 5 × 108 44/76 bacteria/ml (Fig. 1). There was a high degree of concordance between the 10 different serum donors and the level of complement activation related to bacterial concentration. 44/76 and 151/85 activated complement to the same extent (data not shown). Similarly, 44/76 and the 44/76 LPS-deficient mutant activated complement to the same extent (data not shown), consistent with former findings by others (2, 29). Complement activation in whole blood was equal to the activation in serum (data not shown).
FIG. 1.
Complement activation (mean and scatter plot) by twofold increasing concentrations of N. meningitidis in individual serum samples from 10 healthy adults, measured by the soluble TCC level. TCC was significantly increased compared with the basal level at 3 × 107 bacteria/ml and higher, as calculated by one-way ANOVA with repeated measurements and Bonferroni posttest analysis (P < 0.05). AU, arbitrary units; NmB, strain 44/76.
Cytokine secretion in whole blood induced by 44/76, 151/85, and the 44/76 LPS-deficient mutant.
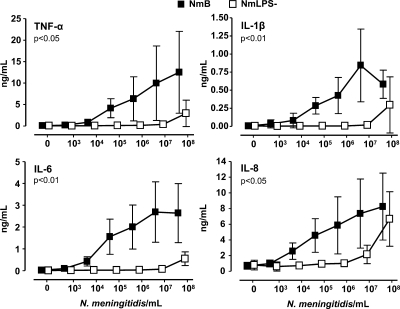
The threshold for secretion of the cytokines TNF-α, IL-1β, IL-6, and IL-8 in whole blood incubated with 44/76 was 103 to 104 bacteria/ml (Fig. 2). Thereafter, the secretion of all cytokines increased dose dependently to 107 bacteria/ml. TNF-α and IL-8 still increased at 108 bacteria/ml, whereas IL-1β fell to a lower concentration and IL-6 was unchanged. Cytokine secretion induced by 151/85 was equal to that induced by 44/76 (data not shown). The threshold for cytokine secretion in whole blood incubated with the 44/76 LPS-deficient mutant was 107 bacteria/ml (Fig. 2). A substantial increase in cytokine secretion was seen when the concentration of the 44/76 LPS-deficient mutant was increased to 108 bacteria/ml.
FIG. 2.
Cytokine secretion (means and standard deviations [SD]) in whole blood from five healthy adults at 10-fold increasing concentrations of wild-type (NmB) and LPS-deficient (NmLPS−) N. meningitidis. P values represent overall differences between the two bacterial strains and were calculated by two-way ANOVA with repeated measurements.
Effect of CD14 inhibition.
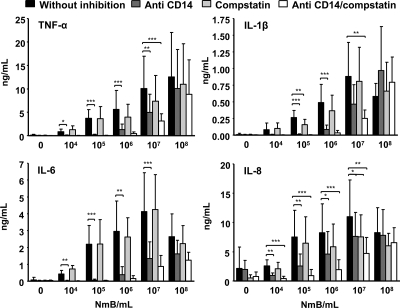
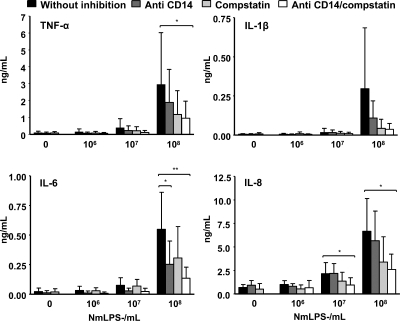
In blood incubated with strain 44/76, anti-CD14 reduced the secretion of all cytokines to baseline levels at ≤105 bacteria/ml (Fig. 3). IL-6 was reduced by 90%, TNF-α and IL-1β were reduced by 80%, and IL-8 was reduced by 60% at 106 bacteria/ml. IL-6 was reduced by 70%, TNF-α and IL-1β were reduced by 50%, and IL-8 was reduced by 35% at 107 bacteria/ml. No effect of CD14 inhibition was seen at 108 bacteria/ml. Increasing the concentration of anti-CD14 up to 10 times (500 μg/ml) in pilot experiments did not give any additional inhibitory effect. Similar results were obtained for blood incubated with 151/85 (data not shown). In blood incubated with the 44/76 LPS-deficient mutant, anti-CD14 reduced the secretion of IL-6 by 60% and that of TNF-α by 40% at 107 bacteria/ml (Fig. 4). IL-1β and IL-6 were reduced by 60%, TNF-α was reduced by 40%, and IL-8 was reduced by 20% at 108 bacteria/ml.
FIG. 3.
Cytokine secretion (means and SD) in whole blood incubated with 10-fold increasing concentrations of wild-type N. meningitidis (NmB). Blood from eight donors was incubated with 105 to 107 bacteria/ml, while blood from five donors was incubated with 104 and 108 bacteria/ml. The effects of inhibiting CD14 (blocking antibody) and complement (C3 level) separately and together were investigated. Statistical significance was calculated at each concentration of meningococci by one-way ANOVA with repeated measurements and Bonferroni posttest analysis. *, P < 0.05; **, P < 0.01; ***, P < 0.001. The significance of the results with combined inhibition is specified only if it is different from that of the results with separate inhibition.
FIG. 4.
Cytokine secretion (means and SD) in whole blood from five healthy adults incubated with 10-fold increasing concentration of LPS-deficient N. meningitidis (NmLPS−). The effects of inhibiting CD14 (blocking antibody) and complement (C3 level) separately and together were investigated. Statistical significance was calculated at each concentration of meningococci by one-way ANOVA with repeated measurements and Bonferroni posttest analysis. *, P < 0.05; **, P < 0.01.
Effects of complement inhibition with compstatin.
In blood incubated with 44/76, compstatin had no effect on cytokine secretion at <105 bacteria/ml (Fig. 3). IL-1β was reduced by 40% at 105 bacteria/ml. TNF-α, IL-1β, and IL-8 were reduced by 25 to 30% at 106 bacteria/ml. TNF-α and IL-8 were reduced by 30% at 107 bacteria/ml. No effect of complement inhibition was seen at 108 bacteria/ml. In blood incubated with 151/85, the effects of complement inhibition with compstatin were similar (data not shown). In blood incubated with the 44/76 LPS-deficient mutant, compstatin inhibited IL-1β, IL-8, and TNF-α by 35 to 50% at 107 bacteria/ml (Fig. 4). IL-1β was reduced by 85%, TNF-α was reduced by 60%, and IL-6 and IL-8 were reduced by 50% at 108 bacteria/ml.
Inhibition of complement by C5aR antagonist.
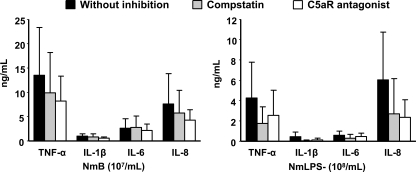
The results for inhibition of complement with a C5aR antagonist did not differ from the results with compstatin, confirming that the complement effect on cytokine secretion was mainly C5aR mediated (Fig. 5).
FIG. 5.
Cytokine secretion (means and SD) in whole blood incubated with wild-type (NmB) and LPS-deficient (NmLPS−) N. meningitidis after inhibition of complement by compstatin (C3 level) versus a C5aR antagonist. No significant differences were found between the two inhibitors by a paired t test.
Cytokine secretion after combined inhibition of CD14 and complement.
In blood incubated with 44/76, combined inhibition using anti-CD14 and compstatin together reduced cytokine secretion more than either of the inhibitors alone at ≥106 bacteria/ml (Fig. 3). TNF-α, IL-1β, and IL-6 were reduced by 95% and IL-8 was reduced by 80% at 106 bacteria/ml. IL-6 was reduced by 80%, IL-1β and TNF-α were reduced by 70%, and IL-8 was reduced by 60% at 107 bacteria/ml. No inhibitory effect was seen at 108 bacteria/ml. Similar results were obtained for blood incubated with 151/85 (data not shown). In blood incubated with the 44/76 LPS-deficient mutant, the combined inhibition reduced the secretion of all cytokines by 50 to 70% at 107 bacteria/ml (Fig. 4). IL-1β was reduced by 90%, IL-6 was reduced by 75%, and IL-8 and TNF-α were reduced by 70% at 108 bacteria/ml.
Activation of NF-κB in transfected HEK293 cells by strain 44/76.
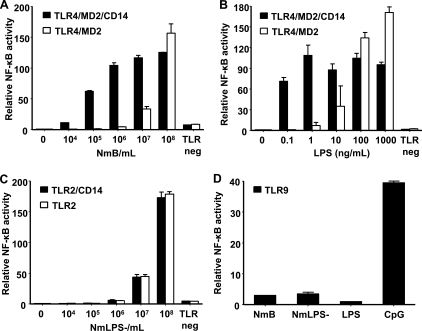
Incubation of HEK293 cells expressing TLR4/MD2 and CD14 upregulated NF-κB activity dose dependently from 104 to 108 bacteria/ml (Fig. 6A). Incubation of cells expressing TLR4/MD2 without CD14 upregulated NF-κB activity dose dependently from 107 to 108 bacteria/ml. NF-κB was equally activated at 108 bacteria/ml, irrespective of the presence of CD14 on the cells. Cells were incubated with medium containing 10% heat-inactivated fetal calf serum. Trace amounts of soluble CD14 (sCD14) could therefore not be excluded, although heat inactivation is supposed to inactivate sCD14. TLR4/MD2-transfected cells were therefore incubated with 108 bacteria/ml under serum-free conditions in a supplementary experiment to exclude any possible effect of sCD14. The cells were activated to the same extent as when 10% fetal calf serum was present in the medium (data not shown). Incubation of cells expressing TLR2 and CD14 upregulated NF-κB activity dose dependently from 107 to 108 bacteria/ml, to the same extent as that for cells expressing only TLR2 (data not shown). Incubation of cells expressing TLR9 induced only negligible upregulation of NF-κB activity (Fig. 6D).
FIG. 6.
Relative upregulation (means and ranges) of NF-κB luciferase activity in HEK293 cells. HEK293 cells expressing TLR4/MD2 alone or in combination with CD14 were incubated with wild-type N. meningitidis (NmB) (A) or purified N. meningitidis LPS (B) at 10-fold increasing concentrations. (C) HEK293 cells expressing TLR2 alone or in combination with CD14 were incubated with the LPS-deficient N. meningitidis strain (NmLPS−). The TLR-negative cells were incubated with 108 bacteria/ml or 1 μg (33,000 EU) LPS/ml. (D) HEK293 cells expressing TLR9 were incubated with 108 bacteria/ml or 1 μg (33,000 EU) LPS/ml.
Activation of NF-κB in transfected HEK293 cells by the 44/76 LPS-deficient mutant.
Incubation of cells expressing TLR2 and CD14 upregulated NF-κB activity dose dependently from 107 to 108 bacteria/ml (Fig. 6C). Incubation of cells expressing only TLR2 upregulated NF-κB activity to the same extent as that for cells expressing TLR2 and CD14. No upregulation of NF-κB activity was seen in cells with TLR4/MD2 or TLR4/MD2 and CD14 (data not shown). Incubation of cells expressing TLR9 induced only negligible upregulation of NF-κB activity (Fig. 6D).
Activation of NF-κB in transfected HEK293 cells by purified N. meningitidis LPS.
Incubation of cells expressing TLR4/MD2 and CD14 upregulated NF-κB activity by purified meningococcal LPS in the range from 100 pg (3.3 EU) to 1 μg (33,000 EU) per ml (Fig. 6B). The responses were almost equal throughout the whole range of LPS concentrations. Incubation of cells expressing TLR4/MD2 without CD14 upregulated NF-κB activity dose dependently from 1 ng (33 EU) LPS/ml to 1 μg (33,000 EU) LPS/ml. NF-κB activity was actually more upregulated in the cells expressing only TLR4/MD2 than in the cells expressing TLR4/MD2 and CD14 at the highest concentrations of LPS. Incubation of cells expressing TLR2 or TLR2/CD14 with LPS had no effect on NF-κB activity (data not shown). Similarly, incubation of TLR9-transfected cells with LPS had no effect (Fig. 6D).
DISCUSSION
Our experiments were designed to study quantitative and cooperative aspects of complement activation and cytokine production induced by increasing loads of N. meningitidis. We found a consistent dose-response relationship for both complement activation and cytokine release with increasing numbers of meningococci. However, the threshold for detectable activation was 3 log higher for complement than for cytokines produced by circulating leukocytes. The association between bacterial load and cytokine secretion is consistent with clinical studies of patients presenting with different stages of meningococcal disease. The levels of TNF-α and IL-1β found in our experiments at 105 meningococci/ml and above were fivefold higher than those found in patients with fulminant meningococcal septicemia (3). The concentrations of IL-8 were about 1 log lower and those of IL-6 were 2 log lower in our whole-blood model than in patients (23, 37, 38). The differences in cytokine levels measured in our model and in patient plasmas are presumably related to cytokine secretion from organs and tissues other than leukocytes during the development of septicemia and to different kinetics of the early inducible cytokines, notably TNF-α. Our whole-blood experiments lasted for 2 hours, whereas the time span from onset of clinical symptoms to hospital admission was a median of 12 h for the patients with septicemia (3, 35).
We know from studies of bactericidal antibodies that the complement system is activated at much lower levels of meningococci than we were able to trace with measurement of soluble TCC in the present experiments (27). A controlled activation of the terminal C5-to-C9 complement pathway leads to insertion of C5b to C9 into the membranes (membrane attack complex) of bacteria and, when efficient, subsequent lysis. Soluble TCC detected in plasma is inactive as a lytic molecule and reflects a spillover when uncontrolled and inappropriate activation of complement takes place. A significant increase of soluble TCC was first detected at 3.2 × 107 meningococci/ml in our assays (Fig. 1). At bacterial levels above this concentration, soluble TCC increased exponentially. We conclude from these experiments that very high levels of meningococci, i.e., 107/ml, are required to reach a state of uncontrolled complement activation. The results are in agreement with results obtained recently by quantifying the levels of circulating meningococci by real-time PCR with plasmas and whole blood from patients with fulminant meningococcal septicemia. These patients are characterized by a bacterial load of a median of 107 meningococci/ml in the circulation and exaggerated complement activation (6, 12, 25). The findings are consistent with the idea that at a certain point, corresponding to a certain amount of bacteria, there is a breakdown of plasma cascade homeostasis. By cross talk between the plasma cascades, the activation of these systems comes to “the point of no return,” when it is impossible to reestablish control of the systems and lethal septic shock develops (19). A similar disturbance of the homeostasis may occur when the bacterial dose increases to a very high level in whole blood, which potentially influences the effects of the various inhibitors discussed below at the highest bacterial concentrations included in this study.
This study demonstrated the role of LPS as the most important stimulus for the cellular innate immune response and, thereby, cytokine secretion elicited by N. meningitidis at a wide range of bacterial concentrations. The effectiveness of a CD14 blocking antibody in downregulating the LPS-induced cytokine response at low to medium concentrations of meningococci was also proved. However, we found that at the upper range of bacterial concentrations (106 to 108 bacteria/ml), CD14 inhibition was less efficient at eliminating the response to LPS. It is unlikely that this result was due to insufficient blocking of CD14, as no additional inhibitory effect was seen when the concentration of CD14 blocking antibody was increased up to 10 times (500 μg/ml) in preliminary experiments. The effect of blocking CD14 also became somewhat more differentiated with respect to the different cytokines as the bacterial concentration was increased, with IL-6 being most dependent on the CD14 pathway. As we further demonstrated, adding a complement inhibitor that prevents the formation of C5a gave an additional inhibitory effect on the secretion of cytokines. Thus, it is tempting to speculate that in order to attenuate the in vivo inflammatory response induced by N. meningitidis, blocking of CD14 may be effective when the bacterial load is low, whereas the addition of a complement inhibitor may be beneficial when the bacterial load increases.
The results of the experiments with 44/76 and 151/85 in whole blood correlated well with the results of the experiments where HEK293 cells expressing TLR4/MD2 or TLR4/MD2 with CD14 were incubated with strain 44/76. Activation of NF-κB in cells expressing TLR4/MD2 was fully dependent on the presence of CD14 at bacterial concentrations of up to 106/ml. The activation was only partly dependent on CD14 at 107 bacteria/ml, and at 108 bacteria/ml the activation of NF-κB was completely independent of CD14. Activation of the same cells with pure LPS was also dependent on CD14 at the lower concentrations but independent of CD14 at the higher concentrations.
Taken together, our results indicate that while CD14 is needed for LPS at low to medium concentrations to stimulate the TLR4/MD2 receptor complex, LPS at high concentrations can stimulate TLR4/MD2 directly, without the involvement of CD14. This is in accordance with findings by others, using macrophages and peripheral blood mononuclear cells (10, 31). Structural variability of the LPS molecule in different bacterial strains seems to influence the ability of LPS to activate TLR4/MD2 independently of CD14, as activation by N. meningitidis LPS has been reported to be less dependent on CD14 than activation by, for example, Escherichia coli LPS (10, 31). Apparently, the primary role of CD14 in meningococcal disease is to facilitate the recognition and reaction to intruding meningococci at the early stage of the bacteremic phase.
Non-LPS membrane structures may also contribute to the cytokine response to meningococci, as shown in the experiments performed with the 44/76 LPS-deficient mutant in this study and consistent with previous studies by others (15, 16, 20, 26, 30). The experiments with whole blood as well as the experiments with HEK293 cells expressing TLR2 or TLR2/CD14 demonstrated that the threshold for stimulation mediated by non-LPS structures with respect to bacterial concentration is high (106 to 107 bacteria/ml). Despite the overall effect of the CD14 blocking antibody and complement inhibition together, some cytokine secretion was still seen in the whole-blood assay with samples challenged with the LPS-deficient N. meningitidis strain. The experiments with HEK293 cells expressing TLR2 or TLR2/CD14 demonstrated clearly that like the TLR4/MD2 receptor, TLR2 can also be activated directly by meningococci, without the aid of CD14.
TLR9 played a negligible role as an activator of NK-κB in our experiments. However, it has been reported that live N. meningitidis cells are more potent than heat-inactivated bacteria in activating TLR9, while no difference was found in the capabilities of live and heat-inactivated meningococci to activate TLR2 and TLR4/MD2 (22).
In conclusion, we have addressed the activation of key components of the innate immune system and their interaction in response to increasing concentrations of N. meningitidis, which are relevant to different clinical presentations of meningococcal disease. We found that the inflammatory response elicited by these systems is highly dependent on the present concentration of meningococci. The threshold for cytokine secretion and activation of NF-κB was 103 to 104 meningococci/ml. At this level, LPS was the sole inflammatory mediator, and activation was dependent on CD14. Activated complement products, mainly C5a, contributed to the inflammatory response when the bacterial concentration was increased to 105 to 106 meningococci/ml. When the concentration was increased further, to 107 bacteria/ml, non-LPS components in the bacterial membrane initiated TLR2-mediated activation, and this increased substantially as the bacterial concentration increased to 108/ml. In parallel with the increasing TLR4 and TLR2 activation, which at this state occurred independently of CD14, the complement activation increased exponentially. Thus, cell activation resulting in cytokine production may clearly occur through different pathways at the most extreme bacterial concentrations observed in patients with fulminant meningococcal sepsis. The combined and inappropriately massive activation of the different systems converts protective defense systems to self-destructive effector mechanisms. We suggest that future therapeutic approaches to meningococcal sepsis, as well as other septic conditions, should aim to target upstream mechanisms of this response.
Acknowledgments
We thank Ernst Arne Høiby and Berit Nyland at the National Health Institute, Oslo, Norway, for providing meningococci; Berit Brusletto at Ullevål University Hospital, Oslo, Norway, for determining bacterial concentrations with RT-PCR; and Are Hugo Pripp, Rikshospitalet University Hospital, Oslo, Norway, for help with statistical analyses. The LPS-deficient N. meningitidis strain (the 44/76 lpxA mutant) was created by Liana Steeghs and Peter van der Ley, National Institute of Public Health and Environment, The Netherlands, and donated to the National Institute of Public Health, Oslo, Norway, for research purposes.
This study was financially supported by The Regional Health Organization Helse Øst, The Research Council of Norway, The Research Council of Rikshospitalet University Hospital, The Family Blix Foundation, Anders Jahre's Fund for the Promotion of Science, and National Institutes of Health grants GM-62134 and AI-068730.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 30 June 2008.
REFERENCES
- 1.Akashi, S., S. Saitoh, Y. Wakabayashi, T. Kikuchi, N. Takamura, Y. Nagai, Y. Kusumoto, K. Fukase, S. Kusumoto, Y. Adachi, A. Kosugi, and K. Miyake. 2003. Lipopolysaccharide interaction with cell surface Toll-like receptor 4-MD-2: higher affinity than that with MD-2 or CD14. J. Exp. Med. 1981035-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjerre, A., B. Brusletto, T. E. Mollnes, E. Fritzsonn, E. Rosenqvist, E. Wedege, E. Namork, P. Kierulf, and P. Brandtzaeg. 2002. Complement activation induced by purified Neisseria meningitidis lipopolysaccharide (LPS), outer membrane vesicles, whole bacteria, and an LPS-free mutant. J. Infect. Dis. 185220-228. [DOI] [PubMed] [Google Scholar]
- 3.Brandtzaeg, P. 2006. Pathogenesis and pathophysiology of meningococcal disease, p. 427-480. In M. Frosch and M. Maiden (ed.), Handbook of meningococcal disease. Wiley-VCH Verlag, Weinheim, Germany.
- 4.Brandtzaeg, P., K. Bryn, P. Kierulf, R. Ovstebo, E. Namork, B. Aase, and E. Jantzen. 1992. Meningococcal endotoxin in lethal septic shock plasma studied by gas chromatography, mass-spectrometry, ultracentrifugation, and electron microscopy. J. Clin. Investig. 89816-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandtzaeg, P., P. Kierulf, P. Gaustad, A. Skulberg, J. N. Bruun, S. Halvorsen, and E. Sorensen. 1989. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J. Infect. Dis. 159195-204. [DOI] [PubMed] [Google Scholar]
- 6.Brandtzaeg, P., T. E. Mollnes, and P. Kierulf. 1989. Complement activation and endotoxin levels in systemic meningococcal disease. J. Infect. Dis. 16058-65. [DOI] [PubMed] [Google Scholar]
- 7.Brandtzaeg, P., R. Ovstebo, and P. Kierulf. 1992. Compartmentalization of lipopolysaccharide production correlates with clinical presentation in meningococcal disease. J. Infect. Dis. 166650-652. [DOI] [PubMed] [Google Scholar]
- 8.Chow, J. C., D. W. Young, D. T. Golenbock, W. J. Christ, and F. Gusovsky. 1999. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 27410689-10692. [DOI] [PubMed] [Google Scholar]
- 9.Finch, A. M., A. K. Wong, N. J. Paczkowski, S. K. Wadi, D. J. Craik, D. P. Fairlie, and S. M. Taylor. 1999. Low-molecular-weight peptidic and cyclic antagonists of the receptor for the complement factor C5a. J. Med. Chem. 421965-1974. [DOI] [PubMed] [Google Scholar]
- 10.Gangloff, S. C., N. Hijiya, A. Haziot, and S. M. Goyert. 1999. Lipopolysaccharide structure influences the macrophage response via CD14-independent and CD14-dependent pathways. Clin. Infect. Dis. 28491-496. [DOI] [PubMed] [Google Scholar]
- 11.Gerard, C. 2003. Complement C5a in the sepsis syndrome—too much of a good thing? N. Engl. J. Med. 348167-169. [DOI] [PubMed] [Google Scholar]
- 12.Hackett, S. J., M. Guiver, J. Marsh, J. A. Sills, A. P. Thomson, E. B. Kaczmarski, and C. A. Hart. 2002. Meningococcal bacterial DNA load at presentation correlates with disease severity. Arch. Dis. Child. 8644-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hackett, S. J., A. P. Thomson, and C. A. Hart. 2001. Cytokines, chemokines and other effector molecules involved in meningococcal disease. J. Med. Microbiol. 50847-859. [DOI] [PubMed] [Google Scholar]
- 14.Hazelzet, J. A., R. de Groot, G. van Mierlo, K. F. Joosten, A. van der Voort, E. Erenberg, M. H. Suur, W. C. Hop, and C. E. Hack. 1998. Complement activation in relation to capillary leakage in children with septic shock and purpura. Infect. Immun. 665350-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ingalls, R. R., E. Lien, and D. T. Golenbock. 2000. Differential roles of TLR2 and TLR4 in the host response to gram-negative bacteria: lessons from a lipopolysaccharide-deficient mutant of Neisseria meningitidis. J. Endotoxin Res. 6411-415. [PubMed] [Google Scholar]
- 16.Ingalls, R. R., E. Lien, and D. T. Golenbock. 2001. Membrane-associated proteins of a lipopolysaccharide-deficient mutant of Neisseria meningitidis activate the inflammatory response through Toll-like receptor 2. Infect. Immun. 692230-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katragadda, M., P. Magotti, G. Sfyroera, and J. D. Lambris. 2006. Hydrophobic effect and hydrogen bonds account for the improved activity of a complement inhibitor, compstatin. J. Med. Chem. 494616-4622. [DOI] [PubMed] [Google Scholar]
- 18.Kawai, T., and S. Akira. 2007. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 13460-469. [DOI] [PubMed] [Google Scholar]
- 19.Markiewski, M. M., B. Nilsson, K. N. Ekdahl, T. E. Mollnes, and J. D. Lambris. 2007. Complement and coagulation: strangers or partners in crime? Trends Immunol. 28184-192. [DOI] [PubMed] [Google Scholar]
- 20.Massari, P., P. Henneke, Y. Ho, E. Latz, D. T. Golenbock, and L. M. Wetzler. 2002. Cutting edge: immune stimulation by neisserial porins is Toll-like receptor 2 and MyD88 dependent. J. Immunol. 1681533-1537. [DOI] [PubMed] [Google Scholar]
- 21.Mathew, S., and G. D. Overturf. 2006. Complement and properidin deficiencies in meningococcal disease. Pediatr. Infect. Dis. J. 25255-256. [DOI] [PubMed] [Google Scholar]
- 22.Mogensen, T. H., S. R. Paludan, M. Kilian, and L. Ostergaard. 2006. Live Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis activate the inflammatory response through Toll-like receptors 2, 4, and 9 in species-specific patterns. J. Leukoc. Biol. 80267-277. [DOI] [PubMed] [Google Scholar]
- 23.Moller, A. S., A. Bjerre, B. Brusletto, G. B. Joo, P. Brandtzaeg, and P. Kierulf. 2005. Chemokine patterns in meningococcal disease. J. Infect. Dis. 191768-775. [DOI] [PubMed] [Google Scholar]
- 24.Mollnes, T. E., T. Lea, S. S. Froland, and M. Harboe. 1985. Quantification of the terminal complement complex in human plasma by an enzyme-linked immunosorbent assay based on monoclonal antibodies against a neoantigen of the complex. Scand. J. Immunol. 22197-202. [DOI] [PubMed] [Google Scholar]
- 25.Ovstebo, R., P. Brandtzaeg, B. Brusletto, K. B. Haug, K. Lande, E. A. Hoiby, and P. Kierulf. 2004. Use of robotized DNA isolation and real-time PCR to quantify and identify close correlation between levels of Neisseria meningitidis DNA and lipopolysaccharides in plasma and cerebrospinal fluid from patients with systemic meningococcal disease. J. Clin. Microbiol. 422980-2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pridmore, A. C., D. H. Wyllie, F. Abdillahi, L. Steeghs, P. van der Ley, S. K. Dower, and R. C. Read. 2001. A lipopolysaccharide-deficient mutant of Neisseria meningitidis elicits attenuated cytokine release by human macrophages and signals via Toll-like receptor (TLR) 2 but not via TLR4/MD2. J. Infect. Dis. 18389-96. [DOI] [PubMed] [Google Scholar]
- 27.Rosenqvist, E., E. A. Hoiby, E. Wedege, K. Bryn, J. Kolberg, A. Klem, E. Ronnild, G. Bjune, and H. Nokleby. 1995. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect. Immun. 634642-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimazu, R., S. Akashi, H. Ogata, Y. Nagai, K. Fukudome, K. Miyake, and M. Kimoto. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 1891777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sprong, T., A. S. Moller, A. Bjerre, E. Wedege, P. Kierulf, J. W. van der Meer, P. Brandtzaeg, M. van Deuren, and T. E. Mollnes. 2004. Complement activation and complement-dependent inflammation by Neisseria meningitidis are independent of lipopolysaccharide. Infect. Immun. 723344-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sprong, T., N. Stikkelbroeck, P. van der Ley, L. Steeghs, L. van Alphen, N. Klein, M. G. Netea, J. W. van der Meer, and D. M. van Deuren. 2001. Contributions of Neisseria meningitidis LPS and non-LPS to proinflammatory cytokine response. J. Leukoc. Biol. 70283-288. [PubMed] [Google Scholar]
- 31.Sprong, T., P. van der Ley, L. Steeghs, W. J. Taw, T. J. Verver-Janssen, M. G. Netea, J. W. van der Meer, and M. van Deuren. 2002. Neisseria meningitidis can induce pro-inflammatory cytokine production via pathways independent from CD14 and Toll-like receptor 4. Eur. Cytokine Netw. 13411-417. [PubMed] [Google Scholar]
- 32.Steeghs, L., H. de Cock, E. Evers, B. Zomer, J. Tommassen, and P. van der Ley. 2001. Outer membrane composition of a lipopolysaccharide-deficient Neisseria meningitidis mutant. EMBO J. 206937-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steeghs, L., R. den Hartog, A. den Boer, B. Zomer, P. Roholl, and P. van der Ley. 1998. Meningitis bacterium is viable without endotoxin. Nature 392449-450. [DOI] [PubMed] [Google Scholar]
- 34.Stephens, D. S., B. Greenwood, and P. Brandtzaeg. 2007. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet 3692196-2210. [DOI] [PubMed] [Google Scholar]
- 35.van Deuren, M., P. Brandtzaeg, and J. W. van der Meer. 2000. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin. Microbiol. Rev. 13144-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Deuren, M., J. van der Ven-Jongekrijg, A. K. Bartelink, R. van Dalen, R. W. Sauerwein, and J. W. van der Meer. 1995. Correlation between proinflammatory cytokines and antiinflammatory mediators and the severity of disease in meningococcal infections. J. Infect. Dis. 172433-439. [DOI] [PubMed] [Google Scholar]
- 37.Waage, A., P. Brandtzaeg, A. Halstensen, P. Kierulf, and T. Espevik. 1989. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J. Exp. Med. 169333-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waage, A., A. Halstensen, and T. Espevik. 1987. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet 1355-357. [DOI] [PubMed] [Google Scholar]
- 39.Ward, P. A. 2004. The dark side of C5a in sepsis. Nat. Rev. Immunol. 4133-142. [DOI] [PubMed] [Google Scholar]