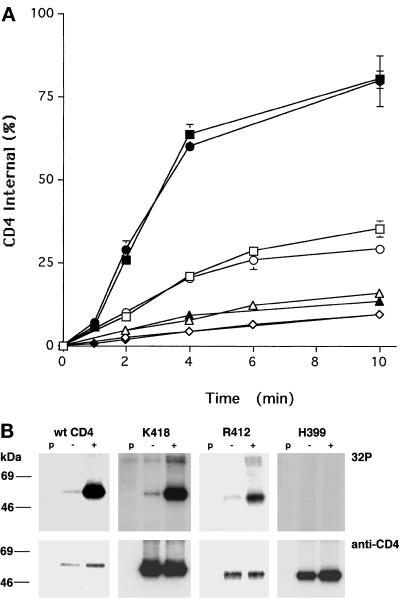
Figure 2.
Endocytosis and phosphorylation of CD4 truncations mutants. (A) HeLa cells stably expressing CD4 or CD4 truncation mutants (Figure 1) were labeled with 0.3 nM 125I-Q4120 at 4°C and then warmed to 37°C in binding medium (open symbols) or medium containing 100 ng/ml PMA (solid symbols) for the indicated times. The total and acid-resistant intracellular radioactivities were determined for each time point as described in MATERIALS AND METHODS. Data from one representative experiment are illustrated, and each point represents the mean ± SD for duplicate samples. □ and ▪, wt CD4; ○ and ●, K418; ▵ and ▴, R412; ⋄ and ♦, H399. (B) Cells were labeled with [32P]orthophosphate (as described in MATERIALS AND METHODS) and incubated in medium with (+) or without (−) 100 ng/ml PMA for 3 min. The cells were rapidly cooled to 4°C and lysed, and the CD4 molecules were immunoprecipitated, separated by SDS-PAGE, and transferred to nitrocellulose paper. CD4 protein was visualized and quantitated by Western blot, and the 32P-activity was determined on the same blots using a phosphorimager. The positions of the molecular weight standards are shown; p, preclear lane (see MATERIALS AND METHODS). Note that the K418 32P lanes illustrated here have been exposed for shorter times than the other panels.