Abstract
The recent sequence analysis of the photosynthetic and plant-symbiotic Bradyrhizobium sp. strain BTAi1 revealed the unexpected presence of a pucBA operon encoding the apoproteins of peripheral light-harvesting (LH) complexes. This pucBA operon is found close to a bacteriophytochrome gene (BphP3B BTAi1) and a two-component transcriptional regulator gene (TFBTAi1 gene). In this study, we show that BphP3B BTAi1 acts as a bona fide bacteriophytochrome and controls, according to light conditions, the expression of the pucBA operon found in its vicinity. This light regulatory pathway is very similar to the one previously described for chromo-BphP4Rp in Rhodopseudomonas palustris and conducts the synthesis of a peripheral LH complex. This LH complex presents a single absorption band at low temperature, centered at 803 nm. Fluorescence emission analysis of intact cells indicates that this peripheral LH complex does not act as an efficient light antenna. One putative function of this LH complex could be to evacuate excess light energy in order to protect Bradyrhizobium strain BTAi1, an aerobic anoxygenic photosynthetic bacterium, against photooxidative damage during photosynthesis.
Anoxygenic phototrophic bacteria have the ability to transform light energy into biochemical amenable energy for their growth and motion. The collection of light and its transformation into chemical energy are mediated by the so-called photosynthetic apparatus. This complex system is composed of three multimeric transmembrane protein complexes: the light-harvesting (LH) complexes, the photochemical reaction center (RC), and the cytochrome bc1 complex located in the intracytoplasmic membrane. Light collected by the peripheral LH complexes is transferred first to the LH1 complex, which absorbs at around 870 nm, and then to the RC, where a charge separation occurs. This initiates a cyclic electron transfer between the RC and cytochrome bc1 via electron carrier proteins in the periplasmic space and quinone molecules in the membrane. This cyclic electron transfer is coupled to the translocation of protons and to the formation of a proton motive force across the inner membrane, ultimately used for ATP synthesis. To optimize light collection, various peripheral LH complexes, coded by different pucBA genes, are expressed according to environmental conditions. In most cases, peripheral LH complexes absorb at 800 and 850 nm and are designated LH2. However, other peripheral LH complexes, which differ by their absorption properties and carotenoid content, have been described. At low light intensities and/or low temperatures, Rhodopseudomonas acidophila synthesizes an LH complex, designated LH3, which absorbs at 800 and 820 nm (4, 8). An atypical LH complex (LH4), presenting a single band around 805 nm, is synthesized by Rhodopseudomonas palustris cells when grown at a low light intensity (16).
In general, the photosynthetic activity of anoxygenic phototrophic bacteria takes place under anaerobic or semiaerobic conditions, since the synthesis of the photosynthetic apparatus is switched on at a low oxygen tension level (5). However, one exception is found for the aerobic anoxygenic phototrophs (AAPs). These bacteria, which are unable to grow in strict anaerobiosis, require oxygen to grow and synthesize their photosynthetic apparatus (32). Shiba et al. (23) were the first to report on the presence of AAPs isolated from seawater, sand, and bottom sediments of Tokyo Bay. Since then, AAPs have been isolated from very diverse ecological niches, including deep-sea hydrothermal vent plume waters and nitrogen-fixing stem nodules (10, 32). The inability to perform an efficient photoinduced cyclic electron transfer under anaerobic conditions is not related to differences in the composition of the photosynthetic apparatus or significant changes in amino acid sequences of LH or RC polypeptides (32). Several explanations have been put forward to explain the growth particularity of AAPs, but the molecular bases underlying this phenomenon are still a matter of debate. Most of the AAPs studied so far possess only the LH1 complex as an antenna. The peripheral LH complexes found in some of the AAPs are distinct from the “classical” LH2 complex of anoxygenic purple bacteria. For example, the absorption maxima of the LH2 complex of Erythromicrobium hydrolyticum, Erythromicrobium ezovicum, and Erythromicrobium ramosum peak at 798 and 832 nm, reminiscent of the LH3 complex (33, 34). The only peripheral LH complex present in Roseobacter denitrificans OCh114, previously named Erythrobacter, exhibits one peak at 806 nm, similar to the LH4 complex of the anaerobic photosynthetic bacterium R. palustris (24).
The recent sequencing of the genomes of the symbiotic AAP Bradyrhizobium strains (ORS278 and BTAi1) revealed the presence of three bacteriophytochrome (BphP) genes in each strain. Two of them (BphP1B and BphP2B) are perfectly conserved and are found in synteny in both strains. The third BphP gene is specific to each strain and is denominated BphP3B BTAi1 or BphP3B ORS278. The product of BphP1B, found in the photosynthesis gene cluster, was previously shown to play a key role in the control of photosystem synthesis in both strains (9, 19). This BphP protein activates the expression of the main photosynthesis genes by antagonizing the action of the repressor PpsR2 (9, 14). The role of the second BphP protein (BphP2B), whose homologs are found in various bacteria (11), remains unknown. No obvious phenotype is observed for the corresponding deletion mutants in Bradyrhizobium ORS278 or R. palustris (unpublished data). A recent study of the specific BphP found in strain ORS278 (BphP3B ORS278) clearly indicates an acquisition by lateral gene transfer (18). BphP3B ORS278 is supposed to control the synthesis of gas vesicles whose genes are found at the vicinity of its gene, but this has not been demonstrated experimentally (18).
The gene of a specific BphP protein of BTAi1 (BBta_3079 or BphP3B BTAi1) is found at the vicinity of a two-component transcriptional regulator gene (BBta_3078) and a putative pucBAC operon (BBta_3081-BBta_3082-BBta_3083) (https://www.genoscope.cns.fr/agc/mage or http://genome.jgi-psf.org/finished_microbes/bra_b/bra_b.home.html). The presence of pucBA genes is surprising, since the synthesis of peripheral LH complexes has not been evidenced so far in this bacterium regardless of the growth mode (i.e., isolation from stem nodules or growth in the laboratory). This genomic organization is also encountered in several strains of R. palustris for which the pucBA.e operon is found close to a BphP gene (BphP4Rp) homolog to BphP3B BTAi1 (31). Interestingly, it has been shown that BphP4Rp acts, depending on the considered strains of R. palustris, either as a light-sensitive (chromo-BphP4Rp) or a redox-sensitive (achromo-BphP4Rp) kinase to control the synthesis of an LH2 complex (31).
By combining biophysical, biochemical, and genetic approaches, we demonstrate in the present study that BphP3B BTAi1 acts as a bona fide BphP protein to control the synthesis of a peripheral LH complex. The ecological bases of this light regulation are discussed.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bradyrhizobium BTAi1 was grown in solid medium on petri dishes under different light conditions as previously described (19). Coillumination of cultures under 700- and 770-nm wavelengths was provided by light-emitting diodes (ELD770-524 and ELD700-524 from Roithner) with an irradiance of 200 μmol and 100 μmol of photons/m2/s, respectively.
Expression and protein procedures.
The entire BphP3B BTAi1 gene was amplified by PCR. Primers designed to add appropriate restriction sites for expression as a His6-tagged version in the pBAD/HisB expression vector (Invitrogen) were used. The 38-amino-acid tag is located at the N terminus of the recombinant protein. This tag contains, in addition to the polyhistidine region, an Xpress epitope and an enterokinase recognition cleavage site that could be used to immunodetect the protein or to remove the tag after purification, respectively. In order to reconstitute BphP3B BTAi1 holo-BphPs in vivo, the hmuO gene from Bradyrhizobium ORS278 was inserted in the above pBAD:BrBphP3.BTAi1 constructs (described in reference 15). The recombinant proteins were overexpressed in Escherichia coli LMG194 and purified as previously described (15). To test the reduction/oxidation of the BphP3B BTAi1 protein, reducing conditions were created with 1 mM dithiothreitol (DTT) and oxidizing conditions with 1 mM K3Fe(CN)6. After addition of the oxidizing or reducing reagent, the protein samples were incubated for at least 30 min on ice and then analyzed by non-reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Protein kinase assays.
Protein kinase reactions were carried out in triplicate as previously described (15). For the phosphotransfer experiments, BphP3B BTAi1 was phosphorylated for 15 min in the presence of [γ-32P]ATP following a 705-nm illumination. This phosphorylation step was followed by a 25-min incubation in the presence of a stoichiometric amount of the Rpa1489 protein (31). 32P-labeled products were quantified by using a Typhoon phosphorimager (Amersham Biosciences).
Construction of BphP3B BTAi1 and pucBAB BTAi1 mutants.
For the construction of the BphP3B BTAi1 null mutant, the gene was introduced into the pJQ200-SK suicide vector (22). The lacZ-Kmr cassette of pKOK5 (20) was then inserted directly into the unique XhoI site of the BphP3B BTAi1 gene. To study the effect of light on pucBA gene expression, the pucBA genes were replaced by the lacZ-Kmr cassette, which contains the lacZ reporter gene. The region flanking pucBA was ligated thanks to the following pairs of primers: up.pucBA.F (5′-CCATGGATCCCATCAGTGTCACGATTTCGGTGAAT-3′) and up.pucBA.R (5′-CAAACTCTGTTGTCGACGTCCGGCATCACGTCACCTCCTGTATTG-3′) and dw.pucBA.F (5′-ATGCCGGACGTCGACAACAGAGTTTGTAGGCGGCGCCGTTCGCGGACAATG-3′) and dw.pucBA.R (5′-CAGGGGCCCCGACAGCATGATCCCGACCAGCGATG-3′) (the primers up.pucBA.R and dw.pucBA.F contain an overlapping sequence of 26 bp [underlined] to facilitate overlap extension PCR). The ligated product was amplified and introduced into the pJQ200-SK plasmid thanks to restriction sites designed in each primer (in bold). The 4.7-kb SalI lacZ-Kmr cassette of pKOK5 was then inserted into the SalI site designed in the overlapping sequences of the up.pucBA.R and dw.pucBA.F primers (in italics). These two constructions were introduced and delivered by conjugation into the BTAi1 strain as previously described (13). Double recombinants were selected on sucrose and confirmed by PCR.
Light action spectrum of pucBA gene expression.
The mutant harboring the lacZ-pucBA fusion was grown under continuous illumination with low irradiance (6.6 μmol·m−1·s−1) of different wavelengths as described previously (19). After growth, the cells under the illuminated area were resuspended in 3 ml of water and β-galactosidase activity was measured as previously described (13).
Absorbance and fluorescence measurements.
Absorption and fluorescence spectra were measured with a Cary 50 spectrophotometer and a Cary Eclipse spectrofluorometer as previously described (15). For both spectrometers, the intensity of the measuring beam (provided by short flashes) is low enough to induce no significant photochemistry. Excitation of purified His-tagged BphP3B BTAi1 was provided by light-emitting diodes emitting at 770 nm or 705 nm (ELD770-524 and ELD 700-524; Roithner) with an irradiance of 15 μmol of photon/m2/s.
Analysis of pucBA and BphP3B BTAi1 genes.
The presence of pucBA and BphP3B BTAi1 genes in different photosynthetic Bradyrhizobium strains was examined by PCR using the following pairs of primers: pucBA.BTAi1.f (5′-GGCAACGCGCTCGCGAGAGTGAAG-3′) and pucBA.BTAi1.r (5′-CTGACCCGATTCATCATCTCTGAAAC-3′) and Br.BphP.BTAi1.f (5′-CCTGGTCCGGTTTCGGCCCAATC-3′) and Br.BphP.BTAi1.r (5′-CTCATGGCCGATCGCCTGCAATC-3′). The localization of the primers is indicated in Fig. 1. A touchdown PCR was done as follows: initial denaturation at 94°C for 5 min, followed by 20 cycles consisting of a 30-s denaturation at 94°C, 30-s annealing temperature from 60 to 50°C, and 1-min primer extension at 72°C, followed by 15 cycles consisting of a 30-s denaturation at 94°C, 30-s annealing temperature at 50°C, and 1-min primer extension at 72°C.
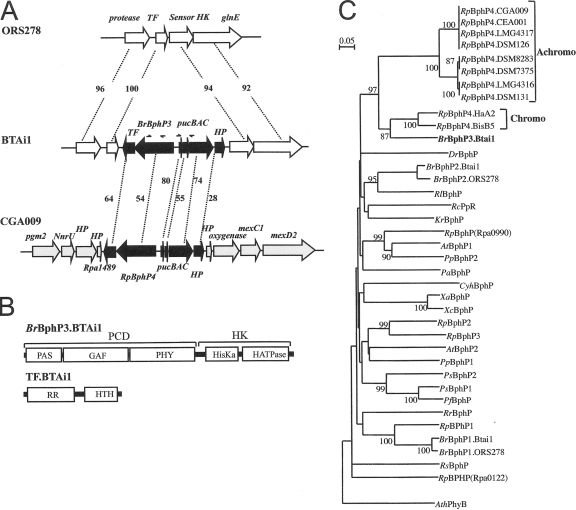
FIG. 1.
Molecular characterization of BphP3B BTAi1. (A) Comparative genomic analysis of the region surrounding the pucBA operon and BphP3B BTAi1 (BrBphP3) in the Bradyrhizobium BTAi1 and ORS278 strains and in R. palustris CGA009. The values given between the genes correspond to the percentages of identity of the corresponding proteins. The arrows indicate the localization of the primers used to search for pucBA and BphP genes in different photosynthetic bradyrhizobia. Abbreviations: TF, transcriptional factor; HP, hypothetical protein. (B) Predicted domain structure of BphP3B BTAi1 (BrBphP3.BTAi1) and TFBTAi1 (TF.BTAi1). HK, histidine kinase domain; HisKa, phosphoacceptor domain; HATPase, ATP binding domain; RR, response regulator domain; HTH, helix-turn-helix domain. (C) Phylogenetic analysis of the BphP family based on an alignment of the GAF domain. The sequences were aligned by using the CLUSTALX software program, and the tree was generated by the neighbor-joining method and displayed using the NJPLOT software program. Bootstrap values, expressed as percentages of 1,000 replications, are given at the branching points. The end points of the GAF domain of each sequence were determined by Pfam analysis (1). Species abbreviations: At, Agrobacterium tumefaciens; Ath, Arabidospsis thaliana; Br, Bradyrhizobium sp.; Dr, Deinococcus radiodurans; Pa, Pseudomonas aeruginosa; Pf, Pseudomonas fluorescens; Pp, Pseudomonas putida; Ps, Pseudomonas syringae; Rc, Rhodospirillum centenum; Rl, Rhizobium leguminosarum; Rp, R. palustris; Rr, Rhodospirillum rubrum; Rs, Rhodobacter sphaeroides; Xa, Xanthomonas axonopodis; Xc, Xanthomonas campestris.
RESULTS
Identification of pucBAC operon close to putative BphP gene in BTAi1 genome.
Comparison of the genomes of two photosynthetic Bradyrhizobium strains (ORS278 and BTAi1), recently annotated, reveals the specific presence of a putative pucBAC operon (BBta_3081-BBta_3082-BBta_3083) in the BTAi1 strain (Fig. 1A). The pucBA genes encode the apoproteins of a peripheral LH complex. The pucC gene is required for a high-level transcription of these genes but also for a proper assembly of LH1 complexes (17). As already mentioned, this pucBAC operon is found close to a putative bphP gene (BBta_3079) and a two-component transcriptional regulator gene (BBta_3078) (Fig. 1A). We specify the products of these two genes as BphP3B BTAi1 and the transcription factor (TF) TFBTAi1, respectively. A similar gene organization was previously found in several R. palustris strains (Fig. 1A).
Pfam analysis by comparison of the protein translation to the Pfam database (1) revealed that BphP3B BTAi1 possesses the classical BphP architecture (Fig. 1B) with a photosensory core domain (PCD) at the N terminus and a C-terminal histidine kinase module involved in signal transduction. TFBTAi1 consists of an N-terminal response regulator domain and a C-terminal helix-turn-helix DNA binding domain (Fig. 1B).
Phylogenetic analysis using the GAF domain of BphP3B BTAi1 (Fig. 1C), a subdomain of the PCD, shows that this BphP is closely related to the BphP4Rp clade (bootstrap value equal to 97%). A similar clustering pattern was obtained using the PHY domain (data not shown). It should be noted that BphP3B BTAi1 displays a higher identity level with chromo-BPhP4Rp proteins (64% with BphP4Rp proteins from the R. palustris HaA2 and BisB5 strains) than with achromo-BphP4Rp (54% with BphP4Rp from CGA009). In agreement, the PCD of BphP3B BTAi1 presents the canonical Cys residue, used by BphP as a bilin attachment site, at position 10, in contrast to achromo-BphP4Rp proteins, which lack this residue. Altogether these data suggest that BphP3B BTAi1 acts as a bona fide BphP protein involved in the control of the pucBAC operon found at its vicinity, as already demonstrated in the case of R. palustris (31).
Expression, purification, and biochemical and biophysical properties of BphP3B BTAi1.
The His-tagged BphP3B BTAi1 protein was coexpressed with a heme oxygenase (required for chromophore synthesis) in E. coli and then purified by affinity chromatography. The absorption spectrum of purified recombinant BphP3B BTAi1 is characteristic of a bona fide BphP (Fig. 2). Similar to the case with chromo-BphP4Rp proteins, BphP3B BTAi1 efficiently binds a chromophore, as determined by fluorescence of the chromoprotein in the presence of zinc and UV light after SDS-PAGE separation (data not shown) (2).
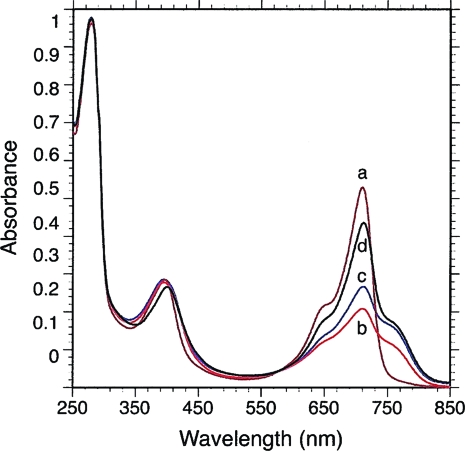
FIG. 2.
Spectral characterization of the His-tagged BphP3B BTAi1 recombinant protein. Absorption spectra of purified BphP3B BTAi1 are as follows: spectrum a (brown line), recorded after 30 min of dark adaptation following a 770-nm preillumination; spectrum b (red line), after 705-nm illumination; spectrum c (blue line), after 2 min of dark after 705-nm illumination; spectrum d (black line), after 30 min of dark after 705-nm illumination.
BphP3B BTAi1 has unusual photochemical properties which are very similar to those observed for chromo-BphP4Rp proteins (31). The dark-adapted state of BphP3B BTAi1 (reached after 30 min of darkness) depends upon the nature of the light it has previously absorbed. When BphP3B BTAi1 is illuminated with infrared light (770 nm) and allowed to adapt to darkness for 30 min, its absorption spectrum is characteristic of the red-absorbing form of a Bph (Pr state) (Fig. 2, spectrum a), with an absorption maximum centered at 710 nm. After illumination with red light (705 nm) and 30 min of dark adaptation, the dark-adapted state is a mixture of Pr and Pfr (far-red-absorbing form) states, which absorbed at 760 and 710 nm, respectively (Fig. 2, spectrum d). Starting from the Pr state (illumination by 770-nm light), red light (705 nm) induces a marked bleaching of this form but the formation of only a small amount of the Pfr state, as shown by minor absorption increase around 760 nm (Fig. 2, spectrum b). This light-induced state (large bleaching of the Pr state and minor formation of the Pfr state) appears similar to the “meta-R” state of Agp1 BphP (3) and phyA (6), a very short-lived intermediate during the dark transition from Pr to Pfr. In BphP3B BTAi1, this state transforms over 2 min to the Pfr state, as shown by the absorption increase around 760 nm (Fig. 2, spectrum c). In fact, the maximum amount of Pfr state is obtained after a 705-nm illumination and a dark adaptation of 5 to 15 min. Conversely, illumination with far-red light induces a rapid and complete formation of the Pr form (not shown). Thus, the peculiar photochemical properties of BphP3B BTAi1 are probably a consequence of a very slow conversion from the intermediate meta-R state to the Pfr state. Steady-state excitation and emission fluorescence measurements show that the Pr state of BphP3B BTAi1 is the main fluorescent state, as observed for other (bacterio)phytochromes (data not shown).
Light signaling pathway of BphP3B BTAi1.
The phosphorylation state of BphP3B BTAi1 was studied under different illumination conditions by in vitro incubation in the presence of [γ-32P]ATP (Fig. 3A). As previously observed for chromo-BphP4Rp proteins (31), BphP3B BTAi1 was maximally phosphorylated in its Pfr form following a 705-nm illumination (Fig. 3A, lane a) while a transition to the Pr state induced a significant decrease in the phosphorylation level (50% ± 7%) (Fig. 3A, lane b). We next addressed the question of the effect of redox conditions on kinase activity of BphP3B BTAi1. No obvious difference in the phosphorylation state of BphP3B BTAi1 was observed after treatment with reducing (DTT) or oxidizing (potassium ferricyanide) agents (Fig. 3B). This is in agreement with the lack of the two Cys residues previously shown to be involved in redox sensitivity of achromo-BphP4Rp proteins (31). Altogether these data indicate that BphP3B BTAi1 acts as a light-regulated histidine kinase.
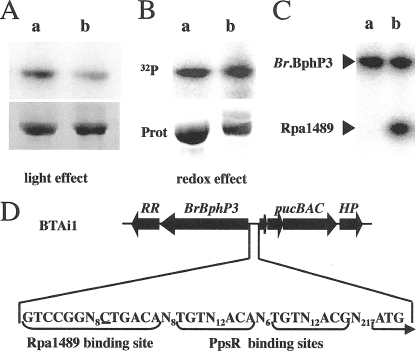
FIG. 3.
BphP3B BTAi1 acts as a light-regulated histidine kinase. (A) Effect of light conditions on kinase activity of BphP3B BTAi1. The chromoprotein was converted preferentially to its Pfr (a) or Pr (b) forms. The maximal amount of the Pfr form was obtained by 15 min of preillumination at 705 nm, followed by 15 min of dark adaptation, and that of the Pr form by illumination at 770 nm. Proteins were incubated with [γ-32P]ATP for 15 min. The reaction products were separated by SDS-PAGE, and the gel was subjected to autoradiography (top) or stained by Coomassie blue (bottom). (B) Effect of redox conditions on kinase activity of BphP3B BTAi1. For the redox effect, the sample was subjected to the following conditions: a 15 min preillumination with 705-nm light, followed by a 15-min dark adaptation; DTT (1 mM) (a) or ferricyanide (1 mM) (b) was used. (C) Phosphotransfer between BphP3B BTAi1 (BrBphP3) and the Rpa1489 recombinant protein. BphP3B BTAi1 was preferentially placed in its Pfr form by a 705-nm illumination followed by 15 min of dark adaptation. Lane a, BphP3B BTAi1 alone; lane b, BphP3B BTAi1 plus Rpa1489. (D) Sequence analysis of the pucBA promoter region of BTAi1 showing the presence of Rpa1489 and PpsR binding sites.
Sequence alignment of the putative transcriptional factor TFBTAi1 from Bradyrhizobium BTAi1 and its homolog, Rpa1489, from R. palustris strain CGA009 revealed a high sequence identity of 64% (Fig. 1A). Unfortunately, the TFBTAi1 recombinant protein was expressed only as inclusion bodies in E. coli. We therefore used its homolog, Rpa1489, to assess the phosphotransfer capability of purified BphP3B BTAi1. As shown in Fig. 3C, we observed an efficient phosphotransfer from BphP3B BTAi1 to purified Rpa1489. This phosphotransfer is maximal when BphP3B BTAi1 is in its Pfr form. Taking into account the high identity between Rpa1489 and TFBTAi1, this suggests a two-component regulatory system where BphP3B BTAi1 is the first element and TFBTAi1 the cognate response regulator.
Expression of pucBA and phenotype of BphP3B BTAi1 deletion mutant.
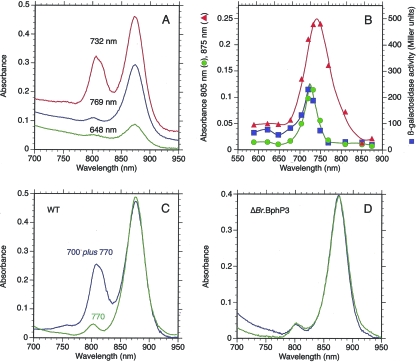
To check the possibility that pucBA is expressed via the pair BphP3B BTAi1/TFBTAi1, we examined the absorption spectra of Bradyrhizobium BTAi1 cells (wild-type [WT] strain) and the expression of β-galactosidase activity in the BTAi1 ΔpucBA mutant grown under illumination of various wavelengths between 590 and 875 nm. Figure 4A shows the absorption spectra of intact cells illuminated at a few selected wavelengths (648, 732, and 769 nm). Whereas illumination with 648- or 769-nm light induces almost exclusively the synthesis of the RC and the LH1 complex, the synthesis of an additional LH complex, absorbing around 810 nm, is observed under 732-nm lighting. The action spectrum of LH1 synthesis (Fig. 4B) is very similar to that reported previously for the related species Bradyrhizobium ORS278 (9). It corresponds to the dark-adapted form of BphP1B BTAi1 (not shown). The synthesis of the additional LH complex is maximal for lights centered at 725 nm (Fig. 4B). A similar action spectrum is obtained for the expression of β-galactosidase activity measured for the BTAi1 ΔpucBA mutant (Fig. 4B), demonstrating that this additional LH complex is the product of the pucBA genes. These action spectra are very similar to the absorption spectrum of BphP3B BTAi1 in its dark-adapted state but slightly shifted to longer wavelengths (725 nm instead of 710 nm). The rationale of this observation is that the synthesis of the additional LH complex requires not only the action of BphP3B BTAi1 but also the activation of BphP1B BTAi1. Indeed, activation of BphP1B BTAi1 induces the synthesis of various enzymes and proteins involved in the synthesis of the photosynthetic apparatus (bch, crt, and puf genes) by antagonizing the repressive activity of PpsR2, which is a prerequisite for the formation of LH complexes. To verify this proposal, WT cells were illuminated with 770-nm light alone or with 700- plus 770-nm light. The 770-nm light, by exciting BphP1B, induces all the enzymes necessary for synthesis of the photosynthetic apparatus, while the 700-nm light preferentially excites BphP3B BTAi1 to induce the transcription of the pucBA genes. Figure 4C compares the absorption spectra of intact cells of Bradyrhizobium BTAi1 grown under these two conditions. While illumination with 770-nm light induces solely the formation of the RC-LH1 complex, the synthesis of an extra LH complex is observed with an additional 700-nm illumination (Fig. 4C). At room temperature, the extra LH complex exhibits an absorption band centered at 805 nm with a shoulder around 815 nm. A definite proof of the essential role played by BphP3B BTAi1 in the synthesis of this additional LH complex is shown by the phenotype of a BphP3B BTAi1 deletion mutant, which does not synthesize this additional LH complex regardless of the illumination conditions (Fig. 4D).
FIG. 4.
BphP3B BTAi1 controls the peripheral LH synthesis in Bradyrhizobium BTAi1. (A) Absorption spectra of Bradyrhizobium BTAi1 cells (WT) grown under semiaerobic conditions, subjected to illumination at various wavelengths (648 nm, green line; 732 nm, red line; 769 nm, blue line). (B) Wavelength dependence of the synthesis of RC/LH1 (red line) or of the peripheral LH complexes (green line) or of the expression of a pucBA-lacZ fusion (blue line). (C) Absorption spectra of Bradyrhizobium BTAi1 cells (WT) grown under 770-nm illumination (green line) or under 700- plus 770-nm light (blue line). (D) Absorption spectra of Bradyrhizobium strain BTAi1 (ΔBphP3B) cells (deletion mutant) grown under 770-nm illumination (green line) or under 700- plus 770-nm lights (blue line).
Distribution of the pucBA operon in photosynthetic Bradyrhizobium strains.
A comparative genomic analysis reveals that the region of 5 kb containing this light regulatory system and the pucBA operon is found in a synteny group of four genes that is perfectly conserved in the ORS278 genome (Fig. 1A) and other rhizobial genomes (not shown). This suggests that this DNA region of the BTAi1 chromosome was acquired by lateral gene transfer, possibly from an R. palustris strain. To strengthen this hypothesis, we searched by PCR for the presence of both the pucBA operon and the BphP3B BTAi1 gene in 107 photosynthetic Bradyrhizobium strains isolated from various countries (Senegal, Mexico, Guyana, etc.). None of them gave PCR products. However, we found the corresponding PCR products for the Blastobacter denitrificans type strain (LMG 8443), a bacterium isolated from a pond in Germany, recently proposed to be renamed Bradyrhizobium denitrificans based on its ability to nodulate Aeschynomene plants and on the phylogenetic analysis of 23S rRNA gene sequences, which implies its grouping with the photosynthetic Bradyrhizobium species, more particularly with strain BTAi1 (29, 30). Sequence analysis of the PCR products of 762 and 772 bp, respectively, confirms a very high identity between these two regions of the Blastobacter denitrificans and BTAi1 genomes: 100% identity for the pucBA operon and only one mismatch with the BphP3B BTAi1 gene. Similar to what we observed for the BTAi1 strain, the expression of the pucBA genes of Blastobacter denitrificans, revealed by a 700-nm illumination, was required for the synthesis of the peripheral LH complex (not shown). Altogether these data indicate that the presence of the peripheral LH complex in photosynthetic bradyrhizobia is very unusual, limited to some rare strains, which acquired this property by lateral gene transfer.
DISCUSSION
Transduction pathway of pucBA operon of Bradyrhizobium BTAi1.
In the present report, we put forward evidence that the third BphP (BphP3B BTAi1) found specifically in the photosynthetic Bradyrhizobium BTAi1 strain is involved in control of the expression of a pucBA operon encoding the apoproteins of an LH complex. Our model is based on the high level of identity observed between BphP3B BTAi1 and BphP4Rp, the conserved arrangement surrounding their genes (Fig. 1A), and the phosphotransfer observed between BphP3B BTAi1 and Rpa1489, a homolog of TFBTAi1. The transcriptional factor Rpa1489 binds to the palindromic motif TGTCCGN8CGGACA that is found on the pucBA.b/e promoter regions of R. palustris strains (31). This promoter site is located downstream from a TGTN12ACA palindrome corresponding to a PpsR binding site, another key regulator of photosynthesis gene expression in purple bacteria (7). Both Rpa1489 and PpsR binding motifs are present in the promoter region of the pucBA operon from Bradyrhizobium BTAi1, as shown in Fig. 3D. The two photosynthetic Bradyrhizobium strains and R. palustris species display the unusual properties of having two distinct PpsRs (encoded by ppsR1 and ppsR2) (19). From these observations, we propose that the pucBA operon from Bradyrhizobium BTAi1 is regulated by a complex regulatory network involving the concerted action of the BphP3B BTAi1/TFBTAi1 two-component system and the PpsR1 and PpsR2 proteins, as already demonstrated for R. palustris strains (31).
Properties and function of the peripheral LH complex of Bradyrhizobium BTAi1.
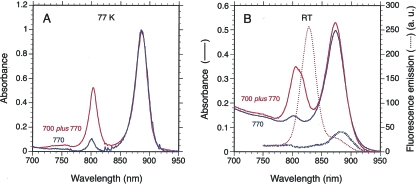
The absorption property of this LH complex at room temperature (Fig. 5B) is very similar to those of the LH3 complex found in R. palustris or R. acidophila. In agreement with this assignment, examination of the amino acid sequence of the pucA product revealed a PhePhe sequence at positions +13 and +14 found in LH3 and LH4 complexes of R. palustris (28). A Thr found at position −5 from the His binding the chromophore is also indicative of an LH3 complex. In addition, the low-resolution structure obtained by Hartigan et al. (16) shows that the Met at the same position in the LH4 complex of R. palustris is a putative ligand of the extra bacteriochlorophyll of this complex, which is absent in the LH3 complex. However, low-temperature measurement of the absorption spectrum of the extra BTAi1 LH complex in intact cells reveals a single band centered at 803 nm, typical of the LH4 complex (Fig. 5A). Therefore, the definite assignment of the peripheral LH complex of BTAi1 as an LH3 or LH4 complex requires further experiments.
FIG. 5.
Absorption and fluorescence emission spectra of intact cells of Bradyrhizobium BTAi1 grown under two different light conditions. (A) Low-temperature (77 K) absorption spectra of intact cells of Bradyrhizobium BTAi1 grown under 770-nm light (blue) or 700- plus 770-nm lights (red). The absorption spectra have been normalized to the same concentration of RCs. (B) Absorption spectra (continuous lines) and emission spectra (dashed lines), recorded at room temperature, of cells grown under 770-nm light or 700- plus 770-nm light are in blue and red, respectively. Recording of the fluorescence emission spectra was performed under 380-nm excitation. The absorption and fluorescence spectra have been normalized to the same concentration of RCs.
The pucA gene product presents a long carboxy-terminal extension of 65 amino acids starting at amino acid 59. BLAST analysis shows that this C-terminal region does not present any similarity to known proteins or functional domains. A search for transmembrane helices based on a hidden Markov model (http://www.cbs.dtu.dk/services/TMHMM/) permits identification of only one helix domain between Thr13 and Leu35, indicating that the C-terminal extension should be located outside the membrane. Such extension has already been observed for the PucA product of several other photosynthetic bacteria. The pucA gene product of Rubrivivax gelatinosus possesses a short alanine- and proline-rich extension, which can be partially reduced without affecting the assembly of the complex (26). On the other hand, the puc2A gene product found in Rhodobacter sphaeroides, which presents a long extension of 209 amino acids, is not functional (35). Of particular interest in the context of the present work is the pucA gene from the AAP Roseobacter denitrificans OCh114, initially named Erythrobacter denitrificans. The recent sequence analysis of the genome of this bacterium revealed that the product of this gene presents an extension of 58 amino acids (27). The corresponding LH presents a single absorption band in the near-infrared region centered around 806 nm, similar to the LH4 complex of R. palustris (16). The molecular masses of the two polypeptides of the LH complex purified from Roseobacter denitrificans OCh114 are around 6 kDa, implying that the PucA polypeptide is processed before insertion into the membrane (24). Several attempts to extract and purify the peripheral LH complex of Bradyrhizobium BTAi1 using various detergents have been unsuccessful. For example, only a supramolecular complex containing both the RC-LH1 complex and the peripheral 805-nm LH complex could be isolated by addition of 1.5% lauryldimethylamine-oxide. Therefore, we do not know presently if the PucA subunit of this peripheral LH complex is assembled in the membrane with or without the extension.
We ascertained the efficiency of energy trapping and transfer to the RC by the peripheral LH complex of Bradyrhizobium BTAi1 by measuring the fluorescence emission spectrum of intact cells of Bradyrhizobium BTAi1 grown under conditions where the peripheral LH complex has been expressed or not (Fig. 5B). In the absence of the peripheral LH complex, the fluorescence emission spectrum peaks around 880 nm, corresponding to fluorescence emitted by LH1 complexes. The fluorescence emission spectrum of membranes containing the peripheral LH complex presents an intense fluorescence emission around 825 nm in addition to that of the LH1 complex. The high yield of the 825-nm fluorescence and the relative low concentration of the peripheral LH complex imply an inefficient energy transfer between these complexes and the LH1 complex. This inefficient energy transfer between these two LH complexes could be related to the small overlap of their associated absorption bands in the near-infrared region. This contrasts with what has been observed for R. palustris, R. acidophila, and Roseobacter denitrificans OCh114, for which an efficient excitonic energy transfer between peripheral LH3/LH4 complexes and LH1 complexes has been reported (4, 8, 25).
Since this peripheral LH complex does not act as an efficient light antenna, what could its physiological role be? As already underlined, the photosystem of AAP bacteria is active only under aerobic conditions. In the presence of oxygen and light, the photosynthetic activity generates triplet states of bacteriochlorophyll molecules. These excited states can react with singlet oxygen and form harmful reactive oxygen species. To cope with this problem, various strains of photosynthetic Bradyrhizobium, symbionts of Aeschynomene, synthesize, in addition to spirilloxanthin, large amounts of canthaxanthin (21). In a previous study, we showed that Bradyrhizobium ORS278 possesses two distinct crt gene clusters for the synthesis of spirilloxanthin and canthaxanthin, respectively (12). While spirilloxanthin is coupled to the photosynthesis activity, canthaxanthin protects the bacteria from oxidative stress (12). The canthaxanthin crt gene cluster, acquired by a lateral gene transfer in Bradyrhizobium ORS278, is absent in Bradyrhizobium BTAi1. One putative function of the peripheral LH complexes of Bradyrhizobium BTAi1, whose genes were also acquired by lateral gene transfer, would also be to evacuate excess light energy in order to protect the bacteria against (photo)oxidative damage during photosynthesis. This hypothesis is reinforced by the fact that the products of the pucBA genes, acquired by lateral transfer probably from R. palustris, have evolved from an LH2-type complex that transfers energy very efficiently to the LH1 complexes to an LH complex, much less efficient in energy transfer.
For both the Bradyrhizobium ORS278 and Bradyrhizobium BTAi1 strains, which photosynthesize under conditions where harmful reactive oxygen species are generated, these two different gene acquisitions may constitute a major selective advantage.
Acknowledgments
Marianne Jaubert and Laurie Vuillet are indebted to the Ministère de l'Education Nationale, de l'Enseignement Supérieur et de la Recherche for a doctoral grant.
Footnotes
Published ahead of print on 7 July 2008.
REFERENCES
- 1.Bateman, A., L. Coin, R. Durbin, R. D. Finn, V. Hollich, S. Griffiths-Jones, A. Khanna, M. Marshall, S. Moxon, E. L. Sonnhammer, D. J. Studholme, C. Yeats, and S. R. Eddy. 2004. The Pfam protein families database. Nucleic Acids Res. 32138-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berkelman, T. R., and J. C. Lagarias. 1986. Visualization of bilin-linked peptides and proteins in polyacrylamide gels. Anal. Biochem. 156195-201. [DOI] [PubMed] [Google Scholar]
- 3.Borucki, B., D. von Stetten, S. Seibeck, T. Lamparter, N. Michael, M. A. Mroginski, H. Otto, D. H. Murgida, M. P. Heyn, and P. Hildebrandt. 2005. Light-induced proton release of phytochrome is coupled to the transient deprotonation of the tetrapyrrole chromophore. J. Biol. Chem. 28034358-34364. [DOI] [PubMed] [Google Scholar]
- 4.Cogdell, R. J., I. Durant, J. Valentine, J. G. Lindsay, and K. Schmidt. 1983. The isolation and partial characterisation of the light-harvesting pigment-protein complexes of Rhodopseudomonas acidophila. Biochim. Biophys. Acta 722427-435. [Google Scholar]
- 5.Drews, G., and J. R. Golecki. 1995. Structure, molecular organization, and biosynthesis of membranes of purple bacteria, p. 725-745. In R. E. Blankenship, M. T. Madigan, and C. E. Bauer (ed.), Anoxygenic photosynthetic bacteria. Kluwer Academic, Dordrecht, The Netherlands.
- 6.Eilfeld, P., and W. Rüdiger. 1985. Absorption spectra of phytochrome intermediates. Z. Naturforsch. 40109-114. [Google Scholar]
- 7.Elsen, S., M. Jaubert, D. Pignol, and E. Giraud. 2005. PpsR: a multifaceted regulator of photosynthesis gene expression in purple bacteria. Mol. Microbiol. 5717-26. [DOI] [PubMed] [Google Scholar]
- 8.Gardiner, A. T., R. J. Cogdell, and S. Takaichi. 1993. The effect of changes in light intensity and temperature on the peripheral antenna of Rhodopseudomonas acidophila. Photosynth. Res. 38159-167. [DOI] [PubMed] [Google Scholar]
- 9.Giraud, E., J. Fardoux, N. Fourrier, L. Hannibal, B. Genty, P. Bouyer, B. Dreyfus, and A. Verméglio. 2002. Bacteriophytochrome controls photosystem synthesis in anoxygenic bacteria. Nature 417202-205. [DOI] [PubMed] [Google Scholar]
- 10.Giraud, E., and D. Fleischman. 2004. Nitrogen-fixing symbiosis between photosynthetic bacteria and legumes. Photosynth. Res. 82115-130. [DOI] [PubMed] [Google Scholar]
- 11.Giraud, E., and A. Verméglio. Bacteriophytochromes in anoxygenic photosynthetic bacteria. Photosynth. Res., in press. [DOI] [PubMed]
- 12.Giraud, E., L. Hannibal, J. Fardoux, M. Jaubert, P. Jourand, B. Dreyfus, J. N. Sturgis, and A. Verméglio. 2004. Two distinct crt gene clusters for two different functional classes of carotenoid in Bradyrhizobium. J. Biol. Chem. 27915076-15083. [DOI] [PubMed] [Google Scholar]
- 13.Giraud, E., L. Hannibal, J. Fardoux, A. Verméglio, and B. Dreyfus. 2000. Effect of Bradyrhizobium photosynthesis on stem nodulation of Aeschynomene sensitiva. Proc. Natl. Acad. Sci. USA 9714795-14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giraud, E., S. Zappa, M. Jaubert, L. Hannibal, J. Fardoux, J.-M. Adriano, P. Bouyer, G. Genty, D. Pignol, and A. Verméglio. 2004. Bacteriophytochrome and regulation of the synthesis of the photosynthetic apparatus in Rhodopseudomonas palustris: pitfalls of using laboratory strains. Photochem. Photobiol. Sci. 3587-591. [DOI] [PubMed] [Google Scholar]
- 15.Giraud, E., S. Zappa, L. Vuillet, J.-M. Adriano, L. Hannibal, J. Fardoux, C. Berthomieu, P. Bouyer, D. Pignol, and A. Verméglio. 2005. A new type of bacteriophytochrome acts in tandem with a classical bacteriophytochrome to control the antennae synthesis in Rhodopseudomonas palustris. J. Biol. Chem. 28032389-32397. [DOI] [PubMed] [Google Scholar]
- 16.Hartigan, N., H. A. Tharia, F. Sweeney, A. M. Lawless, and M. Z. Papiz. 2002. The 7.5-Å electron density and spectroscopic properties of a novel low-light B800 LH2 from Rhodopseudomonas palustris. Biophys. J. 82963-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaschke, P. R., H. N. LeBlanc, A. S. Lang, and J. T. Beatty. 2008. The PucC protein of Rhodobacter capsulatus mitigates an inhibitory effect of light-harvesting 2 and 2 proteins on light-harvesting complex 1. Photosynth. Res. 95279-284. [DOI] [PubMed] [Google Scholar]
- 18.Jaubert, M., J. Lavergne, J. Fardoux, L. Hannibal, L. Vuillet, J.-M. Adriano, P. Bouyer, D. Pignol, E. Giraud, and A. Verméglio. 2007. A singular bacteriophytochrome acquired by lateral gene transfer. J. Biol. Chem. 2827320-7328. [DOI] [PubMed] [Google Scholar]
- 19.Jaubert, M., S. Zappa, J. Fardoux, J.-M. Adriano, L. Hannibal, S. Elsen, J. Lavergne, A. Verméglio, E. Giraud, and D. Pignol. 2004. Light and redox control of photosynthesis gene expression in Bradyrhizobium: dual roles of two PpsR. J. Biol. Chem. 27944407-44416. [DOI] [PubMed] [Google Scholar]
- 20.Kokotek, W., and W. Lotz. 1989. Construction of a lacZ-kanamycin-resistance cassette, useful for site-directed mutagenesis and as a promoter probe. Gene 84467-471. [DOI] [PubMed] [Google Scholar]
- 21.Lorquin, J., F. Molouba, and B. L. Dreyfus. 1997. Identification of the carotenoid pigment canthaxanthin from photosynthetic Bradyrhizobium strains. Appl. Environ. Microbiol. 631151-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quandt, J., and M. F. Hynes. 1993. Versatile suicide vectors which allow direct selection for gene replacement in gram-negative bacteria. Gene 12715-21. [DOI] [PubMed] [Google Scholar]
- 23.Shiba, T., U. Simidu, and N. Taga. 1979. Distribution of aerobic bacteria which contain bacteriochlorophyll a. Appl. Environ. Microbiol. 3843-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimada, K., H. Hayashi, and M. Tasumi. 1985. Bacteriochlorophyll-protein complexes of aerobic bacteria, Erythrobacter longus and Erythrobacter species OCh114. Arch. Microbiol. 143244-247. [Google Scholar]
- 25.Shimada, K., H. Hayashi, T. Noguchi, and M. Tasumi. 1990. Excitation and emission spectroscopy of membranes and pigment-protein complexes of an aerobic photosynthetic bacterium, Erythrobacter sp. Och114. Plant Cell Physiol. 31395-398. [Google Scholar]
- 26.Steunou, A.-S., S. Ouchane, F. Reiss-Husson, and C. Astier. 2004. Involvement of the C-terminal extension of the alpha polypeptide and of the PucC protein in LH2 complex biosynthesis in Rubrivivax gelatinosus. J. Bacteriol. 1863143-3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swingley, W. D., S. Sadekar, S. D. Mastrian, H. J. Matthies, J. Hao, H. Ramos, C. R. Acharya, A. L. Conrad, H. L. Taylor, L. C. Dejesa, M. K. Shah, M. E. O'huallachain, M. T. Lince, R. E. Blankenship, J. T. Beatty, and J. W. Touchman. 2007. The complete genome sequence of Roseobacter denitrificans reveals a mixotrophic rather than photosynthetic metabolism. J. Bacteriol. 189683-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tharia, H. A., T. D. Nightingale, M. Z. Papiz, and A. M. Lawless. 1999. Characterisation of hydrophobic peptides by RP-HPLC from different spectral forms of LH2 isolated from Rhodopseudomonas palustris. Photosynth. Res. 61157-167. [Google Scholar]
- 29.van Berkum, P., and B. D. Eardly. 2002. The aquatic budding bacterium Blastobacter denitrificans is a nitrogen-fixing symbiont of Aeschynomene indica. Appl. Environ. Microbiol. 681132-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Berkum, P., J. M. Leibold, and B. D. Eardly. 2006. Proposal for combining Bradyrhizobium spp. (Aeschynomene indica) with Blastobacter denitrificans and to transfer Blastobacter denitrificans (Hirsch and Muller, 1985) to the genus Bradyrhizobium as Bradyrhizobium denitrificans (comb. nov.). Syst. Appl. Microbiol. 29207-215. [DOI] [PubMed] [Google Scholar]
- 31.Vuillet, L., M. Kojadinovic, S. Zappa, M. Jaubert, J. M. Adriano, J. Fardoux, L. Hannibal, D. Pignol, A. Verméglio, and E. Giraud. 2007. Evolution from light to redox sensing for a bacteriophytochrome. EMBO J. 263322-3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yurkov, V., and J. T. Beatty. 1998. Aerobic anoxygenic phototrophic bacteria. Microbiol. Mol. Biol. Rev. 62695-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yurkov, V., N. Gad'on, A. Angerhofer, and G. Drews. 1994. Light-harvesting complexes of aerobic bacteriochlorophyll-containing bacteria Roseococcus thiosulfatophilus, RB3 and Erythromicrobium ramosum, E5 and the transfer of excitation energy from carotenoids to bacteriochlorophyll. Z. Naturforsch. 49579-586. [Google Scholar]
- 34.Yurkov, V., E. Stackebrandt, O. Buss, A. Verméglio, V. Gorlenko, and J. T. Beatty. 1997. Reorganization of the genus Erythromicrobium: description of “Erythromicrobium sibiricum” as Sandaracinobacter sibiricus gen. nov., sp. nov., and of “Erythromicrobium ursincola” as Erythromonas ursincola, gen. nov., sp. nov. Int. J. Syst. Bacteriol. 471172-1178. [DOI] [PubMed] [Google Scholar]
- 35.Zeng, X., M. Choudhary, and S. Kaplan. 2003. A second and unusual pucBA operon of Rhodobacter sphaeroides 2.4.1: genetics and function of the encoded polypeptides. J. Bacteriol. 1856171-6184. [DOI] [PMC free article] [PubMed] [Google Scholar]