FIG. 2.
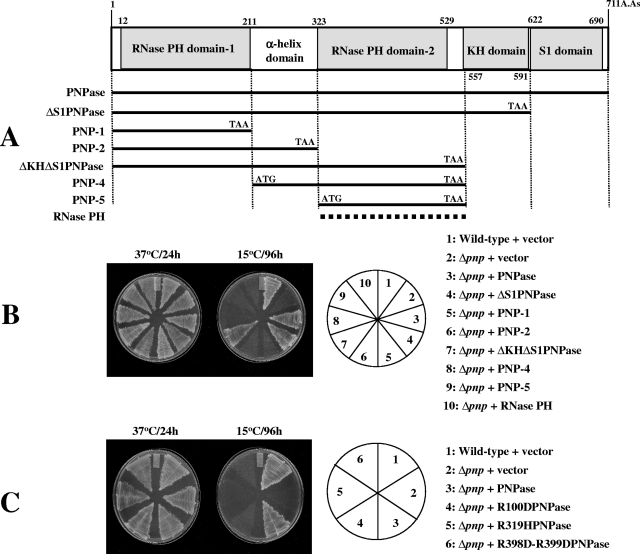
RNase activity of PNPase is critical for its cold shock function. (A) Schematic representation of domains of PNPase and plasmids constructed. Different domains of PNPase were cloned in the pINIII vector using NdeI and BamHI sites, as shown. The RNase PH plasmid shown with a dashed line is the pINIII plasmid carrying rph, the gene encoding E. coli RNase PH. (B) E. coli wild-type cells were transformed with the pINIII plasmid as a control, and Δpnp cells were transformed with the pINIII plasmid alone or the pINIII plasmid containing either the entire PNPase, different domains of PNPase, or RNase PH. The plasmids carrying different domains of PNPase or RNase PH are designated as in panel A. The cells were streaked on LB plates containing ampicillin (50 μg ml−1) and incubated at 37°C or 15°C. Results for plates incubated at 37°C for 24 h or at 15°C for 96 h are presented. (C) E. coli wild-type cells were transformed with the pINIII plasmid as a control, and Δpnp cells were transformed with the pINIII plasmid alone or the pINIII plasmid containing either the wild-type PNPase or PNPase carrying the R100D, R319H, or R398D-R399D mutations. The cells were streaked on LB plates containing ampicillin (50 μg ml−1) and incubated at 37°C or 15°C. Results for plates incubated at 37°C for 24 h or at 15°C for 96 h are presented.