Abstract
Feline immunodeficiency virus (FIV) is the Lentivirus responsible for an immunodeficiency-like disease in domestic cats (Felis catus). FIV is divided into five phylogenetic subtypes (A, B, C, D, and E), based on genetic diversity. Knowledge of the geographical distribution of subtypes is relevant for understanding different disease progressions and for vaccine development. In this study, viral sequences of 26 infected cats from Rio de Janeiro, 8 undergoing treatment with zidovudine (AZT) for at least 5 years, were successfully amplified from blood specimens. gag capsid (CA), pol reverse transcriptase (RT), and env gp120 (V3-V4) regions were analyzed to determine subtypes and to evaluate potential mutations related to antiretroviral drug resistance among treated cats. Subtyping based on phylogenetic analysis was performed by the neighbor-joining and maximum likelihood methods. All of the sequences clustered with subtype B in the three regions, exhibiting low genetic variability. Additionally, we found evidence that the same virus is circulating in animals in close contact. The analysis of FIV RT sequences identified two new putative mutations related to drug resistance located in the RT “finger” domain, which has 60% identity to human immunodeficiency virus (HIV) sequence. Amino acid change K→R at codons 64 and 69 was found in 25% and 37.5% of the treated animals, respectively. These signatures were comparable to K65R and K70R thymidine-associated mutations found in the HIV-1 HXB2 counterpart. This finding strongly suggests a position correlation between the mutations found in FIV and the K65R and K70R substitutions from drug-resistant HIV-1 strains.
Feline immunodeficiency virus (FIV) is the Lentivirus that infects members of Felidae family. FIV isolates from domestic cats have been detected all over the world. Five pure subtypes of FIV (A, B, C, D, and E) and their mosaic forms have been identified, based on analysis of V3-V5 env gene and p25 gag gene (12, 28, 33, 39). Subtypes A and B are the most common worldwide isolates. Subtype A has been reported in the United States, Canada, Europe, Australia, and New Zealand (3, 8, 11, 33, 34, 39, 41). Subtype B has been reported in the United States, Canada, Europe, and Japan (3, 11, 17, 29, 33). Subtype C has been reported in the United States, Canada, Europe, New Zealand, Taiwan, and Vietnam (3, 11, 15, 27, 33). Subtype D has been reported in Japan and Vietnam (17, 27), and subtype E has been reported in Argentina (28). The prevalence of FIV-positive animals varies among countries, depending on the groups of animals tested. Among sick cats tested, some authors found about FIV prevalences of 47% in cats in England (6), 43.9% in cats in Japan (16), and 52.7% in cats in France (26). There are few data about the prevalence of FIV-positive domestic cats in Brazil—mostly from studies of sick cats done in veterinary clinics which detected FIV infection in 14% of infected cats in São Paulo (32) and 37.5% of infected cats in Rio Grande do Sul (5). Overall rates of infection of cats were 2.7% in Minas Gerais (7) and 16.66% in Rio de Janeiro (40) in random sampling studies with sick and healthy cats.
The infection in domestic cats is characterized by a long period without symptoms, followed by increased susceptibility to opportunistic infections leading to AIDS-like disease. A vaccine based on subtypes A and D was developed in 2002 in an attempt to control new infections (45). When the animal is already infected, one possibility to reduce viral replication is antiretroviral (ARV) therapy. This ARV therapy is based on nucleoside reverse transcriptase (RT) inhibitors (NRTIs) such as zidovudine (AZT) and lamivudine (3TC). These therapies can lead to immunological and clinical improvement and may prolong the life expectancy of treated animals. Drug classes that inhibit human immunodeficiency virus type 1 (HIV-1) have been tested on FIV, and the real effectiveness is still controversial (1). FIV is not inhibited by non-NRTIs and protease inhibitors but responds to NRTIs and, more recently, to integrase strand-transfer inhibitors (36). The emergence of drug resistance mutants in infected cats is believed to result in clinical failure of antiviral therapy, based on AIDS treatment knowledge (19). In the 1990s, some virus resistance mutations associated with ARV in cats were characterized by in vitro assays. FIV RT mutations V47I (51), M183T/V (37), P156S (38), and D3H (24) were described. There have been no reports in the literature evaluating in vivo resistance mutations in cats receiving ARV for a long period of time. More information about RT gene resistance mutations in the FIV model is relevant, and it could be useful as an ARV therapy model for human AIDS treatment.
In this work, we describe the genetic profiles of 26 FIV isolates infecting domestic cats in Rio de Janeiro, Brazil. We found that 100% of the isolates were subtype B, based on phylogenetic analysis targeting three different coding regions throughout the FIV genome: p25 capsid (CA), RT, and the V3-V4 loop of envelope. Some of the cats were undergoing NRTI-based ARV therapy, and the genotypic profiles of drug resistance were also analyzed for the pol RT region.
MATERIALS AND METHODS
Study population.
Samples were collected between May 2006 and August 2007 from different locations at Rio de Janeiro city and vicinity. The locations were different selected areas represented herein by letters A to E, where locations A to D represent samples from Rio de Janeiro and location E represents samples from Niterói, a city about 13 km away from Rio de Janeiro. All 26 cats were rescued from the street: 23 cats were in private shelters, and 3 were individually rescued and adopted by different people. Eleven cats came from location A, 3 from location B, 10 from location C, and 1 from location D, and 1 kitten was from location E. Neighborhoods A and B are adjacent. Eight cats from location A had started the NRTI treatment by the time they arrived at the shelter (Table 1).
TABLE 1.
Clinical characterization of samplesa
| Sample | Collection date (mo/yr) | Sex | Neighborhood | Location | ARV treatment
|
Clinical symptom(s)b | Outcome | |
|---|---|---|---|---|---|---|---|---|
| 1st (mo/yr begins-ends) | 2nd (mo/yr begins) | |||||||
| RJ01 | 5/06 | M | Vila Isabelc | A | Clinically healthy | |||
| RJ02 | 5/06 | M | Vila Isabelc | A | Clinically healthy | |||
| RJ03 | 7/06 | M | Vila Isabel | A | AZT (5/02-2/07) | AZT + 3TC (3/07) | Clinically healthy | |
| RJ04 | 7/06 | M | Vila Isabel | A | AZT (11/00-2/07) | AZT + 3TC (3/07) | Clinically healthy | |
| RJ05 | 7/06 | M | Vila Isabel | A | AZT (9/01-2/07) | AZT + 3TC (3/07) | Clinically healthy | |
| RJ06 | 7/06 | F | Vila Isabel | A | AZT (11/00-2/07) | AZT + 3TC (3/07) | Clinically healthy | |
| RJ07 | 6/06 | M | Guaratiba | C | MI, URTI | |||
| RJ08 | 6/06 | F | Guaratiba | C | MI, URTI, pyothorax | Death 2/07 | ||
| RJ09 | 6/06 | M | Guaratiba | C | MI, URTI, mycoplasma | Death 8/06 | ||
| RJ10 | 6/06 | M | Guaratiba | C | MI, URTI | |||
| RJ11 | 6/06 | M | Guaratiba | C | MI, URTI, opisthotonus | |||
| RJ12 | 6/06 | F | Guaratiba | C | MI, URTI | Death 3/07 | ||
| RJ13 | 6/06 | F | Guaratiba | C | MI, URTI | |||
| RJ14 | 6/06 | M | Guaratiba | C | MI, URTI | |||
| RJ15 | 6/06 | F | Guaratiba | C | MI, URTI | |||
| RJ16 | 6/06 | M | Guaratiba | C | MI, URTI | |||
| RJ17 | 6/06 | M | Tijucac | B | Clinically healthy | |||
| RJ18 | 7/06 | F | Vila Isabel | A | AZT (11/00-6/06) | Anemia, diarrhea | Death | |
| RJ19 | 9/06 | F | Niteróid | E | URTI, seizures, mycoplasma, anemia | |||
| RJ20 | 10/06 | M | Alto Boa Vista | D | URTI | |||
| RJ21 | 9/06 | M | Vila Isabel | A | Abscess, cachexia | Death 9/06 | ||
| RJ22 | 10/06 | F | Vila Isabel | A | AZT (11/00-2/07) | AZT + 3TC (3/07) | Clinically healthy | |
| RJ23 | 10/06 | F | Vila Isabel | A | AZT (11/00-11/06) | Neoplasy | Euthanized | |
| RJ24 | 10/06 | F | Vila Isabel | A | AZT (11/00-2/07) | AZT + 3TC (3/07) | Clinically healthy | |
| RJ34 | 7/07 | M | Tijuca | B | Cutaneous infection, myiasis | |||
| RJ35 | 9/07 | M | Tijuca | B | Diarrhea | |||
Shown are the isolate names, date of total blood collection for this study, domestic cat sex (M, male; F, female), neighborhoods where the animals were captured, classification of place according to neighborhood borders, date of first NRTI therapy introduction and end of regimen, date of second NRTI therapy introduction, clinical symptoms presented by each animal on the date of sample collection, and clinical outcome.
Neurological disorders: MI, motor incoordination; URTI, upper respiratory tract infection.
Cat that was adopted after capture.
Kitten.
Sample collection.
Whole blood from 26 domestic cats, positive for FIV in serological tests, was drawn with 3-ml sterile EDTA tubes. Serum and peripheral blood mononuclear cells (PBMCs) were separated by centrifugation and stored at −80°C until use. A second sample draw was performed with animals RJ03, RJ04, RJ05, RJ06, RJ10, RJ13, and RJ24 to confirm the similarity between samples and discard the possibility of contamination. The genomic DNA from 200 μl of each whole-blood sample was extracted with QIAamp DNA blood mini kit (Qiagen, Germany), according to the manufacturer's protocol. The viral RNA was extracted from 140 μl of collected plasma using the QIAamp viral RNA mini kit (Qiagen, Germany), only for samples from cats RJ08, RJ09, RJ12, and RJ18. These plasma samples were used to confirm the sequence profile and discount contamination problems. New samples from these cats were not possible as they died sometime after the first blood sample withdrawal.
Nested PCR amplification and sequencing.
Primers were designed based on sequence of isolate TM2 (subtype B) from Japan. Nested PCR outer and inner primers were made to amplify DNA coding sequences of p25 CA, RT, and the V3-V4 loop of envelope. The nested PCR fragments generated were 405, 603, and 554 bp, respectively (Table 2).
TABLE 2.
List of primers used in nested PCR and in sequencing reaction
| Region and primera | Sequence (5′→3′) | Length | Terminal position
|
|
|---|---|---|---|---|
| 5′ | 3′ | |||
| CA | ||||
| Gag out CA F | GACTGTATCTACTGCCACAGC | 21-mer | 927 | 947 |
| Gag out CA R | TTCTTGGCAGGCCCTCAGTTTT | 22-mer | 1674 | 1653 |
| Gag int CA F | GGGATTAGACACCAGACCATC | 21-mer | 972 | 992 |
| Gag int CA R | CTGGGCTCTGCTTGTTGTTCT | 21-mer | 1376 | 1356 |
| V3-V4 | ||||
| Env out F | GCGCAAGTAGTGTGGAGACT | 20-mer | 6791 | 6810 |
| Env out R | CTGCATAACTTCTTCCGGCAC | 21-mer | 8080 | 8060 |
| Hd int F | GGATGGTGGAACCAAGTAGC | 20-mer | 7334 | 7353 |
| Env int R | GGTAAATCCGATGTGCAAGACC | 22-mer | 7887 | 7866 |
| RT | ||||
| RT out F | GGAGTAGGAGGAGGAAAAAGAGGAAC | 26-mer | 2157 | 2182 |
| RT out R | GCCCATCCACTTATATGGGGGC | 22-mer | 3029 | 3008 |
| RT int F | GGGCCTCAGGTAAAACAGTGGC | 22-mer | 2391 | 2412 |
| RT int R | GTCTTCCGGGGTTTCAAATCCCCAC | 25-mer | 2993 | 2969 |
The PCR conditions were 1.25 U of Taq polymerase Platinum (Invitrogen), 2.5 mM MgCl2, 1× Taq Platinum buffer (Invitrogen), 0.2 mM of each deoxynucleoside triphosphate (dNTP), and 1.25 pmol of each primer to a final volume of 50 μl, using 5 μl of extracted genomic DNA (DNAg; eluted volume from extraction, 50 μl). The first round of reaction performed for DNAg was 98°C for 5 min, followed by 35 cycles of 95°C for 40 s, 55°C for 45 s, and 72°C for 90 s, with one final cycle of 72°C for 7 min; an ABI9700 Thermocycler (Applied Biosystems) was used. For nested PCRs and the first round using cDNA, the cycling conditions were 95°C for 5 min, followed by 35 cycles of 95°C for 40 s, 56°C for 45 s, and 72°C for 60 s, with one final cycle of 72°C for 7 min. The PCR was purified with Montage PCR centrifugal filter devices, according to the manufacturer's protocol. The material was quantified by comigration into a 1% agarose Tris-borate-EDTA gel with a DNA mass ladder (low-DNA-mass ladder; Invitrogen), before being submitted to the sequencing reaction.
Each primer used for the second round of amplification was also used separately for sequencing of each FIV genomic region. The sequencing was performed by an “in-house” methodology using the ABI big dye terminator v.3.1 kit and ABI3100 sequencer (Applied Biosystems).
Virus subtyping and sequence analysis.
The sequences generated were manually edited with SeqMan software. Primer locations were excluded for the analysis: fragment lengths obtained to final analysis were 362 bp for CA, 554 bp for RT, and 487 bp for the V3-V5 regions. Each region was aligned with reference sequences available at the GenBank website using the ClustalW tool (44). The phylogenetic analysis of all the sequences was made by using the neighbor-joining method performed with MEGA 3.1 software (18). The distance matrix was generated by Kimura's two-parameter model for nucleotides. The statistical strength of the neighbor-joining method was assessed by bootstrap resampling (1,000 replicates). To build the trees of CA and RT, we used as the outgroup a sequence from Pallas' cat (Otocolobus manul), FIVOMA. To calculate the overall genetic distances of each subtype group and between groups, we used Kimura's two-parameter model in MEGA 3.1 software. The pairwise matrix was generated and then plotted in a graph in Sigmaplot 9.0 software.
To determine the substitution models that would best fit our data sets for maximum likelihood (ML) analysis, program Modeltest 3.06 (30) was performed which gives the best model for the hierarchical likelihood ratio test (hLRTs) (42) and for the Akaike information criterion (AIC). The models suggested for gag, pol, and env by the hLRTs were HKY+I+G, K81uf+G, and TrN+I+G, respectively. For AIC, the models selected were HKY+I+G, TrN+I+G, and GTR+I+G. The ML trees were inferred using PAUP*, version 4.0b10 (43). All models suggested were tested, with the exception of K81uf+G, which was substituted for by GTR+G. The TrN+I+G and the GTR+G scripts were generously provided by Artur Trancoso Lopo de Queiroz, and the other scripts were obtained from the Bioafrica.net website (M. Salemi and T. de Oliveira, PAUP* scripts and tutorials section at http://www.bioafrica.net). ML phylogenetic trees constructed with the models suggested by hLRT are available in the supplemental material.
The following subtype-specific reference sequences of FIV, as well as the outgroup sequence from Pallas' cat, were downloaded from GenBank (accession numbers given in parentheses): subtype A, Petaluma (M25381), PPR (M36968), and Sendai1 (D37820 and D37813); subtype B, isolate from the United States, USIL2489_7B (U11820), isolates from Italy, M2 (X69501) and M3 (X69502), and isolates from Japan, TM2 (M59418), Sendai 2 (D37821 and D37814), Aomori 1 (D37823 and D37816), Aomori 2 (D37824 and D37817), and Yokohama (D37819 and D37812); subtype C, isolates from Canada, C (AF474246) and C36 (AY600517), and isolates from Taiwan, Ti1 (AB027298) and Ti3 (AB027300); subtype D, isolates Fukuoka (D37822 and D37815) and Shizuoka (D37818 and D37811); and subtype E, isolates LP3 (AB027302 and D84496), LP20 (AB027303 and D84498), and LP24 (AB027304 and D84500). The accession number for the selvage isolate from Pallas' cat is AY713445.
Nucleotide sequence accession numbers.
All sequences generated in this work have been submitted to GenBank. Their accession numbers are EU375620 to EU375645 for the CA gag region, EU375568 to EU375593 for the RT pol region, and EU375594 to EU375619 for the V3-V4 env region.
RESULTS
Sampling of infected cats.
In this study, 26 samples from FIV-positive domestic cats (Felis catus) were collected from five different sites in Rio de Janeiro state (Table 1). There were 16 males and 10 females, with 1 female kitten. From 11 cats that were rescued in location A (Vila Isabel neighborhood), 3 have been adopted and 8 were maintained in a private shelter. The latter eight cats had been undergoing treatment with AZT starting from 2000 to 2002. One cat died in July 2006, and another was euthanized due to cancer. In March 2007, the six remaining animals undergoing AZT monotherapy had their ARV treatment switched to AZT (5 mg) plus 3TC (4 mg/kg of body weight) twice a day due to the availability of a capsule formulation by that time. Those cats undergoing treatment were clinically healthy from the beginning of therapy until the submission of the manuscript for this article. At location C (Guaratiba neighborhood), 10 positive animals were selected: all of them presented with motor incoordination and upper respiratory tract infection. In this group, four cats have died within the last 2 years.
For locations less represented, we found one infected cat living in a shelter at location D and one infected female kitten adopted by a citizen at location E. An infected cat was identified at location B, where two other cats belonging to the same group were rescued and adopted by a veterinary clinic and another person. All detailed clinical symptoms are presented in Table 1.
Sample amplifications.
All 26 samples collected were submitted to genomic DNA extraction, and the three regions (p25 CA, RT, and the V3-V4 loop of envelope) from the genomic provirus were successfully amplified by nested PCR as described, and sequenced for further genetic and phylogenetic analyses.
All samples that showed 100% similarity in any region (samples from cats RJ06, RJ08, RJ09, RJ10, RJ12, RJ13, RJ18, RJ34, and RJ35) were submitted to a new PCR from the first PCR round, and the amplicon product was sequenced.
The new sequencing result showed again a profile of 100% similarity, and we performed a new genomic DNA extraction for sequencing again. This was only possible for samples from cats RJ08, RJ09, RJ10, RJ12, and RJ13, from which we still kept preserved PBMCs. The results remained the same.
To confirm this profile and avoid PBMC contamination and recover material (PBMCs) from those not yet reevaluated, we performed a new blood collection from cats who provided samples RJ06, RJ10, and RJ13, followed by extraction of DNAg, PCR, and sequencing. The cats who provided samples RJ08, RJ09, RJ12, and RJ18 died during the study, and we could not recover any material postmortem. Cats RJ34 and RJ35 were street cats, and we could not assess again their material. For those samples analyzed, the results continued to be the same.
For previously collected samples from animals already dead, we performed the RNA extraction from plasma and than sequenced the cDNA (viable virus; samples from cats RJ08, RJ09, RJ12, and RJ18). In this case, the samples also had the same profile found before.
NRTI-treated cats RJ03, RJ04, RJ05, RJ22, and RJ24 were also submitted to this new round of blood collection sequencing, to confirm their genomic profiles.
Virus subtyping.
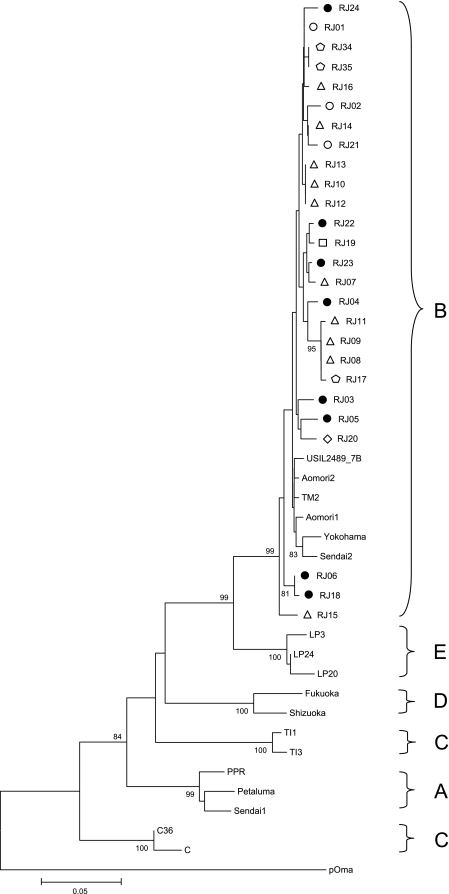
Genetic analysis of the gag (CA) region comprised 362 bp (positions 994 to 1355 relative to isolate TM2). Phylogeny showed that all samples clustered with subtype B sequences previously described and presented low genetic variability (Fig. 1). An average of 2% genetic variability within all 26 samples from Brazil (see Fig. 4A) was found. Some samples collected at the same location showed 100% intralocal similarity in the CA: samples from cats RJ08 and RJ09 at location C; RJ10, RJ12, and RJ13 at location C; and RJ34 and RJ35 at location B. To confirm this genetic profile and discount for contamination, new samples were collected, and the extraction and PCRs were performed on different days. The profiles remained exactly the same. All samples analyzed did not clustered monophyletically but were able to group together with all subtype B reference sequences (99% of bootstrap value at the defining node), possibly reflecting a high degree of intraclade variability. Three samples (from cats RJ15, RJ06, and RJ18), whose isolates diverged significantly from the other clade B isolates, were analyzed for an original founder event of recombination between different subtypes. For that purpose, we sequenced a seminested fragment of 660 nucleotide pairs for RJ15 (using outer reverse and inner forward PCR primers) to perform recombination analysis in Simplot, version 3.5.1, software (20). The 660-bp fragment did not show any evidence of recombination with known non-B subtypes (data not shown).
FIG. 1.
Phylogenetic tree from 362 nucleotides of CA gag alignment. The tree was inferred using the neighbor-joining distance method and Kimura's two-parameter model. Shown are bootstrap values of 1,000 replicates. Bootstrap values greater than 70% are shown. Circles represent isolates from location A (black circles represent cats undergoing treatment), pentagons represent isolates from location B, triangles represent isolates from location C, lozenges represent isolates from location D, and squares represent isolates from location E. The horizontal bar indicates the nucleotide substitution scale.
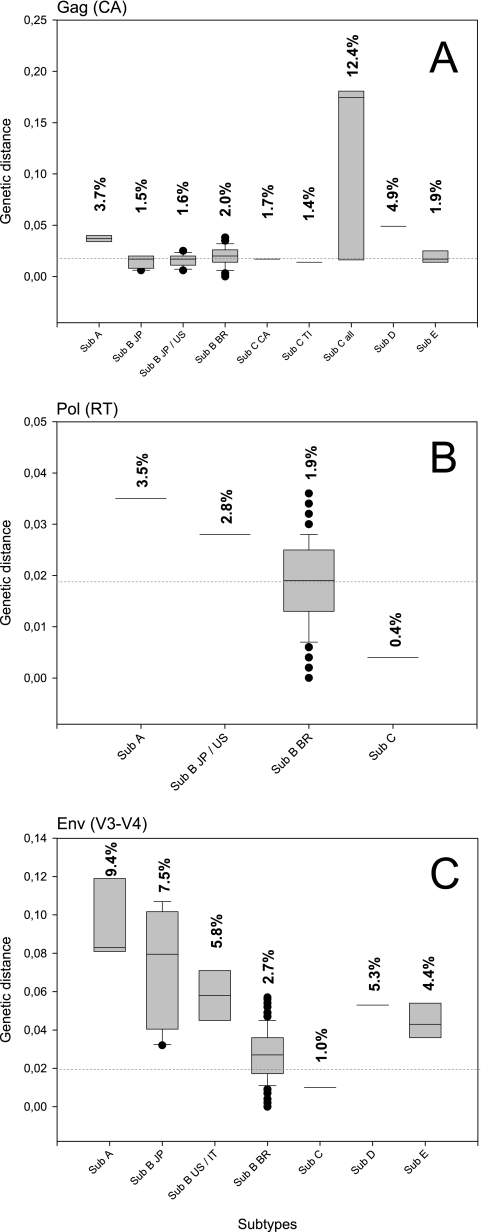
FIG. 4.
Box plot representation of genetic distances found within each subtype group analyzed. Values above the bars represent the overall mean of genetic distance found in each group. Dashed lines indicate 2.0% genetic distance. The sequence isolates used are shown in each box plot. (A) CA region. The following samples were used: subtype A (Sub A), Petaluma, PPR, and Sendai1; subtype B from Japan (Sub B JP), Sendai2, TM2, Yokohama, Aomori1, and Aomori2; subtype B from Japan and from the United States (Sub B JP/US), Sendai2, TM2, Yokohama, Aomori1, Aomori2, and USIL2489_7B; subtype B from Brazil (Sub B BR), RJ01, RJ02, RJ03, RJ04, RJ05, RJ06, RJ07, RJ08, RJ09, RJ10, RJ11, RJ12, RJ13, RJ14, RJ15, RJ16, RJ17, RJ18, RJ19, RJ20, RJ21, RJ22, RJ23, RJ24, RJ34, and RJ35; subtype C from Canada (Sub C CA), C and C36; subtype C from Taiwan (Sub C Ti), Ti1 and Ti3; subtype C from Canada and Taiwan (Sub C all), C, C36, Ti1, and Ti4; subtype D (Sub D), Shizuoka and Fukuoka; and subtype E (Sub E), LP20E, LP24E, and LP3E. (B) RT region. The following samples were used: Sub A, Petaluma and PPR; Sub B JP/US, TM2 and USIL2489_7B; Sub B BR, RJ01, RJ02, RJ03, RJ04, RJ05, RJ06, RJ07, RJ08, RJ09, RJ10, RJ11, RJ12, RJ13, RJ14, RJ15, RJ16, RJ17, RJ18, RJ19, RJ20, RJ21, RJ22, RJ23, RJ24, RJ34, and RJ35; subtype C from Canada (Sub C), C and C36. (C) V3-V4 region. The following samples were used: Sub A, Petaluma, PPR, and Sendai1; Sub B JP, Sendai2, TM2, Yokohama, Aomori1, and Aomori2; subtype B from the United States and Italy (Sub B US/IT), M2, M3, and USIL2489_7B; Sub B BR, RJ01, RJ02, RJ03, RJ04, RJ05, RJ06, RJ07, RJ08, RJ09, RJ10, RJ11, RJ12, RJ13, RJ14, RJ15, RJ16, RJ17, RJ18, RJ19, RJ20, RJ21, RJ22, RJ23, RJ24, RJ34, and RJ35; subtype C from Canada (Sub C), C and C36; Sub D, Shizuoka and Fukuoka; and Sub E, LP20E, LP24E, and LP3E.
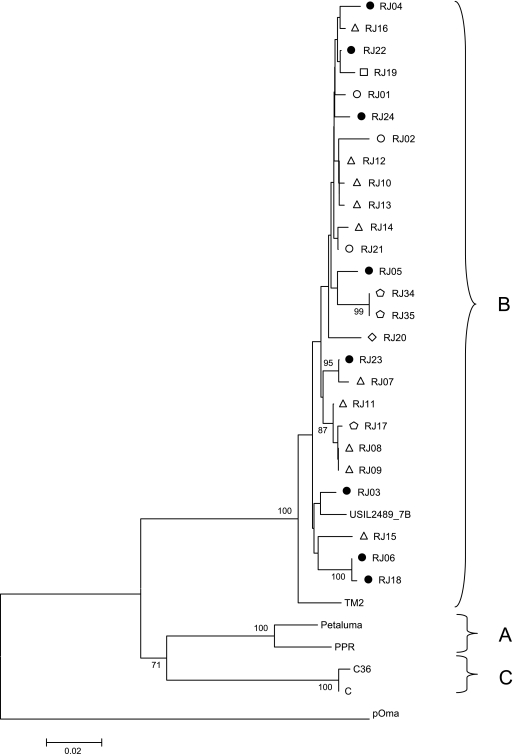
Genetic analysis of the pol (RT) region was comprised of 554 bp (positions 2415 to 2968 relative to isolate TM2). We found an average of 1.9% genetic variability within all 26 samples from Brazil (Fig. 4B). Phylogeny analysis showed that all samples also clustered with the only two subtype B reference isolates available for this genomic region: USIL2489_7B from the United States and TM2 from Japan (Fig. 2). Nevertheless, Japanese isolate TM2 positioned as a clade B outlier, different from the North American reference sequence USIL2489_7B, although it still kept a bootstrap value of 100% with the clade B cluster. As well as in the gag CA region, samples from cats RJ08 and RJ09 (location C) and RJ34 and RJ35 (location B) from the same geographical region showed 100% similarity. This was not observed for isolates from cats RJ10, RJ12, and RJ13 (location C), which were identical for the gag CA region previously analyzed. To discount again the possibility of contamination, the same procedure was followed as described for gag CA region reanalysis.
FIG. 2.
Phylogenetic tree from 554 nucleotides of RT pol alignment. The tree was inferred as described in the legend to Fig. 1. The symbols used in this figure represent different regions and treatment statuses as depicted in Fig. 1.
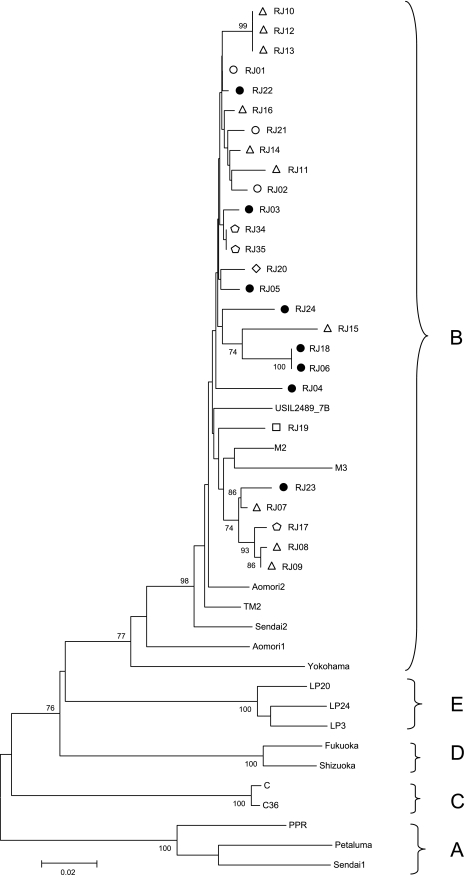
Genetic analysis of env (V3-V4) region was comprised of 487 bp (positions 7358 to 7844 related to isolate TM2). Phylogeny showed that all Brazilian samples clustered again as subtype B sequences, being more related to references M2 and M3 from Italy and USIL2489_7B from the United States, than to the Japanese references (Fig. 3). The cluster of Brazilian sequences presented 2.7% of mean genetic variability within all 26 samples from Brazil (Fig. 4B). Samples collected at the same location that showed 100% similarity at the V3-V4 region were from cats RJ10, RJ12, and RJ13 at location C (samples also identical at gag CA region); cats RJ34 and RJ35 at location B (samples also identical at the gag CA and pol RT regions); and cats RJ06 and RJ18 at location A. Samples from cats RJ08 and RJ09 (location C) that were shown to be identical to other regions analyzed were not identical at the env V3-V4 region.
FIG. 3.
Phylogenetic tree from 487 nucleotides of V3-V4 env alignment. The tree was inferred as described in the legend to Fig. 1. The symbols used in this figure represent different regions and treatment statuses as depicted in Fig. 1.
The best tree topologies found were made using neighbor joining with Kimura's two-parameter model. The analysis of ML trees didn't change the results of Brazilian sample subtyping, confirming that all samples in all regions belonged to subtype B. For this reason, these trees generated according to hLRT models are available as supplemental data (see Fig. SA1, SA2, and SA3 in the supplemental material).
Genetic distance analysis.
FIV isolates showed relative low genetic variability: about 2.0% in CA, 1.9% in RT, and 2.7% in the V3-V4 region (Fig. 4). In contrast, we observed intrasubtype variabilities of 3.7%, 3.5%, and 9.4% at the respective regions in subtype A sequences from GenBank; variability of about 12.4% in CA for subtype C; and variability of 7.5% in the V3-V4 region of subtype B from Japan, as well as 5.8% genetic variability in the V3-V4 region within subtype B samples from the United States and Italy (Fig. 4C).
When intersubtype sequences were compared, Brazilian samples showed the highest genetic similarity to samples of subtype B to U.S. samples, as revealed by the pairwise genetic distance analysis (Tables 3, 4, and 5). In CA, only 2% distance was observed from a subtype B prototype from the United States (USIL2489_7B); 3% distance was observed from subtype B samples from Japan and the United States (TM2 and USIL2489_7B), and 4.4% distance was observed from subtype B samples from the United States and Italy (M2, M3, and USIL2489_7B).
TABLE 3.
Pairwise distance matrix between groups of subtypes for the CA region
| CA subtypea | % Distance
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Sub A | Sub B JP | Sub B BR | Sub C | Sub C TI | Sub D | Sub E | Pallas' cat | |
| Sub A | ||||||||
| Sub B JP | 17.20 | |||||||
| Sub B BR | 17.90 | 2.20 | ||||||
| Sub C | 16.30 | 20.20 | 19.80 | |||||
| Sub C TI | 18.70 | 18.10 | 17.40 | 16.90 | ||||
| Sub D | 18.60 | 17.70 | 17.10 | 18.60 | 16.30 | |||
| Sub E | 17.40 | 9.20 | 9.20 | 19.60 | 19.40 | 15.70 | ||
| Pallas' cat | 32.90 | 41.40 | 39.70 | 30.80 | 34.30 | 36.70 | 38.10 | |
| Sub B US | 17.20 | 1.40 | 2.00 | 19.80 | 17.40 | 17.00 | 9.10 | 41.00 |
Sub A, subtype A (Petaluma, PPR, and Sendai1); Sub B JP, subtype B from Japan (Sendai2, TM2, Yokohama, Aomori1, and Aomori2); Sub B US, subtype B from the United States (USIL2489_7B); Sub B BR, subtype B from Brazil (RJ01, RJ02, RJ03, RJ04, RJ05, RJ06, RJ07, RJ08, RJ09, RJ10, RJ11, RJ12, RJ13, RJ14, RJ15, RJ16, RJ17, RJ18, RJ19, RJ20, RJ21, RJ22, RJ23, RJ24, RJ34, and RJ35); Sub C, subtype C from Canada (C and C36); Sub C TI, subtype C from Taiwan (Ti1 and Ti3); Sub D, subtype D (Shizuoka and Fukuoka); Sub E, subtype E (LP20E, LP24E, and LP3E); Pallas' cat, Pallas' cat selvage isolate.
TABLE 4.
Pairwise distance matrix between groups of subtypes for the RT region
| RT subtypea | % Distance
|
|||
|---|---|---|---|---|
| Sub A | Sub B BR | Sub B JP/US | Sub C | |
| Sub A | ||||
| Sub B BR | 14.10 | |||
| Sub B JP/US | 15.00 | 3.00 | ||
| Sub C | 12.10 | 14.80 | 15.70 | |
| Pallas' cat | 24.80 | 26.00 | 25.20 | 26.00 |
Sub A, subtype A (Petaluma and PPR), Sub B JP/US, subtype B from Japan and from the United States (TM2 and USIL2489_7B); Sub B BR, subtype B from Brazil (RJ01, RJ02, RJ03, RJ04, RJ05, RJ06, RJ07, RJ08, RJ09, RJ10, RJ11, RJ12, RJ13, RJ14, RJ15, RJ16, RJ17, RJ18, RJ19, RJ20, RJ21, RJ22, RJ23, RJ24, RJ34, and RJ35); Sub C, subtype C from Canada (C and C36); Pallas' cat, Pallas' cat selvage isolate.
TABLE 5.
Pairwise distance matrix between groups of subtypes for the V3-V4 region
| V3-V4 region subtypea | % Distance
|
|||||
|---|---|---|---|---|---|---|
| Sub A | Sub B JP | SubB US/IT | Sub B BR | Sub C | Sub D | |
| Sub A | ||||||
| Sub B JP | 19.90 | |||||
| SubB US/IT | 22.80 | 7.10 | ||||
| SubB BR | 20.70 | 5.80 | 4.40 | |||
| Sub C | 20.80 | 18.50 | 19.50 | 17.50 | ||
| Sub D | 21.80 | 16.40 | 18.00 | 16.40 | 21.10 | |
| Sub E | 22.50 | 16.60 | 17.80 | 15.90 | 20.90 | 18.00 |
Sub A, subtype A (Petaluma, PPR, and Sendai1); Sub B JP, subtype B from Japan (Sendai2, TM2, Yokohama, Aomori1, and Aomori2); Sub B US/IT, subtype B from the United States and from Italy (M2, M3, and USIL2489_7B); Sub B BR, subtype B from Brazil (RJ01, RJ02, RJ03, RJ04, RJ05, RJ06, RJ07, RJ08, RJ09, RJ10, RJ11, RJ12, RJ13, RJ14, RJ15, RJ16, RJ17, RJ18, RJ19, RJ20, RJ21, RJ22, RJ23, RJ24, RJ34, and RJ35); Sub C, subtype C from Canada (C and C36); Sub D, subtype D (Shizuoka and Fukuoka); Sub E, subtype E (LP20E, LP24E, and LP3E).
Genotypic profile of resistance to ARV.
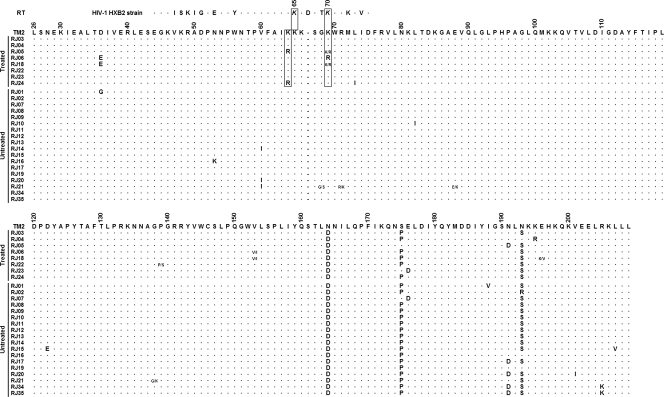
The 26 sequences analyzed were separated into two groups of origin: cats that were undergoing ARV treatment (8 cats) and ARV treatment-naïve cats (18 cats). The pol RT sequences from virus isolates were aligned and searched for the presence of ARV-resistance mutations as previously described (24, 37, 38, 51). No V47I, P156S, or M183T/V resistance mutations in RT were found (Fig. 5). The fragments analyzed begin at amino acid 26 of RT, excluding the substitution D3H from this analysis. All mutations that were present in at least two treated isolates and were not present in any other naïve isolate were taken into consideration for genotypic analysis of resistance. Amino acid changes D35E and V153I were present in 25% of the treated isolates, but also appeared in some subtype A, C, and Pallas' cat reference sequences, where those polymorphisms appear to be subtype-specific molecular signatures.
FIG. 5.
Alignment of RT pol by position related to TM2 genome. Shown are positions 2415 to 2968 (554 bp; amino acids 26 to 209). For the HXB2 HIV-1 isolate shown at the top, positions K65R and K70R of HIV-1 are highlighted in italics and boxed with their FIV RT counterparts. Amino acids are represented by the IUPAC one-letter code. Dots in the alignment represent similar amino acids to the TM2 sequence at each position. Dashes represent sequence gaps (related to the HIV-1 HXB2 sequence). Numbers over the sequences represent the actual position of the amino acid in the original RT sequence. Ambiguities at certain positions of a single sequence are shown by two amino acids separated by a dash.
We found K64R in 25% and K69R mutations in 37.5% of the treated samples. Arginine at positions 64 and 69 was not found in any isolates from the treated naïve cats, other subtype reference sequences, or the Pallas' cat selvage isolate. Those two substitutions arose together in isolate RJ05 from the AZT-treated group, with K64R also found in RJ24 and K69R in RJ06 and RJ18 isolates, the same two isolates carrying the subtype-specific polymorphisms D35E and V153I described herein. Statistical analysis using a two-tailed (double one-tailed) Fisher's exact test showed a significant correlation between ARV treatment in cats and the appearance of K64R and/or K69R (P = 0.0094, α = 0.05), inferring a possible role of those mutations in the phenotypic resistance to nucleoside analogs used during ARV therapy on those cats.
When the FIV RT sequences were compared to that of HIV-1 sequence isolate HXB2 (accession no. K03455), we found high similarity around these positions in both sequences (Fig. 5). This FIV region, encompassing amino acids K64, K65, K66, and K69 of the conserved KKK(X)nK domain, correlates with HIV-1 RT positions K64, K65, K66, and K70. In HIV, Ky→R substitutions at positions 65 and 70 of RT are strongly related to cross-resistance to nucleoside analogs and AZT resistance, respectively.
DISCUSSION
Brazilian FIV strains and their epidemic spread have not been deeply characterized. FIV subtype B was detected in one sample based on gag sequence in a brief report from Minas Gerais state (7). A recently published review indicated that subtype E was the only one prevalent in Brazil (49). In our study, classification of 26 samples based on sequencing analysis of viral genomic regions gag, pol, and env identified B as the only subtype circulating in FIV-positive animals in Rio de Janeiro State (Rio de Janeiro city and Niterói city). Our findings are in accordance with the report from Caxito et al. (7) in Minas Gerais state. Additionally, we tested sequences from Brazilian isolates available at the GenBank database (unpublished data) which represent two different states: São Paulo and Minas Gerais. All available sequences of gag and env from Brazilian isolates previously characterized clustered with our subtype B strains herein described as well as with international reference sequences of subtype B (data not shown). These findings show evidence that subtype B is the only subtype detected in Brazil, and the epidemiologic information generated herein contributes to the knowledge of the diversity of FIV strains circulating in this country. This knowledge will be instrumental for testing the efficacy of commercial vaccine available with subtypes A and D (Fel-O-Vax FIV).
Here we found that the subtype B circulating in Rio de Janeiro exhibits low genetic variability (about 2.0% in CA, 1.9% in RT, and 2.7% in V3-V4) in comparison to other subtypes, such as subtype A (3.7%, 3.5%, and 9.4% variability for CA, RT, and V3-V4, respectively) (Fig. 4). These findings contrast with those of a previous report in which the env gene was found to be less diverse in subtype A than in subtype B (3). Although apparently discordant from this latter work, the lower subtype B diversity from Brazilian isolates just reflects a regional characteristic of recent genetic dispersion from founder events of FIV infections occurring in Brazil (such as the infections analyzed from cats at the same shelter), compared to the high divergence among the control group sequences with no apparent infection case correlation and from different countries.
In the CA region, subtype C was the most variable one, showing 12.4% distance when samples from Canada and Taiwan were analyzed (Fig. 4A). The virus circulating in animals from Rio de Janeiro state exhibited relative homogeneity within isolates from the same location. In location A, two samples had 100% similarity in the V3-V4 region. In location B, two isolates (from cats RJ34 and RJ35) had 100% similarity in all regions analyzed. These animals lived in close contact and probably were infected with the same virus. Also, these animals were captured at the same ground property. Analysis of full-length genome is necessary to confirm this hypothesis. Finally at location C, a shelter with about 1,400 domestic cats (∼200 FIV-positive animals), we found two isolates sharing 100% similarity in the CA and RT regions, and three other isolates showed the same similarity in CA and V3-V4 regions. Although other genomic regions analyzed did not present the same 100% similarity found in some regions of these isolates' genomes (discounting clonal contamination), these data suggest the homogeneity of these closely related isolates as a result of the recent infection between cats living together in the same shelter.
At collection day, clinical symptoms of 26 animals included in this study varied from clinically healthy to neurological disorders. Of note, there is no correlation between ARV treatment and clinical symptoms.
In the group of treated animals, the viral isolates did not show any RT mutation (V47I, P156S, and M183T/V) previously described as associated with ARV resistance (37, 38, 51). Nevertheless, we found two new putative mutations associated with ARV resistance: positions K64R and K69R were present in 25% (2/8) and 37.5% (3/8) of the NRTI-treated group, respectively. The domestic cats had been undergoing treatment for at least 5 years; this long period of treatment contributed to the selection of resistance mutation-carrying strains, and this fact enhances the chance to detect resistance mutations. These two substitutions resemble their HIV counterparts K65R and K70R (Fig. 5), which are located at the RT “finger” domain. These mutations are related to the HIV RT loss of sensitivity to 3TC, stavudine, didanosine, zalcitabine, abacavir, emtricitabine, tenofovir, and AZT. In HIV-1, K65R is associated with a low-multidrug-resistance mechanism and resistance to 3TC, stavudine, didanosine, zalcitabine, abacavir, emtricitabine, and tenofovir (9, 10, 21, 22, 25, 46, 47, 50). The mutation K70R in HIV-1 is associated with a high level of resistance to AZT and stavudine, contributing to the RT capacity of pyrophosphorolysis (2). Also, K70R was detected as the first mutation that appears with prolonged AZT therapy in humans (4). Although this finding indicates a possible role of these two substitutions (K64R and K69R) in the FIV resistance to NRTIs, further studies should support these resistance genotypes through resistance phenotyping assays and mutant reversion by site-directed mutagenesis followed by resistance phenotyping of generated viruses carrying these mutations separately and together. Due to the results shown above, we suggest that K64R and K69R found in treated cats are new putative drug-associated mutations in FIV playing the role of K65R and K70R from HIV-1.
When the region near residues 64 and 69 of RT is compared to that in HIV-1 strain HXB2, we find high similarity between sequences. It is already known that HIV-1 and FIV RTs differ in their length by 1 or 2 amino acids, corresponding to residues G112 and S68 or T69 in HIV-1 (31). Here we find that FIV has 1 amino acid less than HIV-1 in position D67 of RT: the region 63-KKKDSTK-70 of HIV-1 corresponds to 64-KKKSGK-69 in FIV. Surprisingly, the deletion of residue D67 (Δ67 RT mutation) in HIV is strongly related to resistance to AZT and is usually followed by the substitution ST→SG in the subsequent amino acids (positions 68 and 69 in HIV-1 RT) (13, 14, 23, 35, 48). This exact profile was found in all FIV sequences analyzed in this work, as well as in all the FIV RT reference sequences from elsewhere. Whether this FIV RT molecular signature represents a natural genetic barrier to the effectiveness of some NRTIs (mainly AZT) still needs to be addressed by further studies using mutant reversion by site-directed mutagenesis followed by a drug resistance phenotyping assay.
This work shows an innovative genetic analysis using gag, pol, and env sequences simultaneously. This methodology can give a better insight into the evolution of FIV circulating in an urban setting. The use of long-term-treated cats can also lead to better comprehension of the RT gene sequence, like the finding of new clinically relevant thymidine analog mutations in FIV.
Supplementary Material
Acknowledgments
We thank the veterinarians Sidney Jiro Nohara and Paulo Roberto Lima de Menezes for help with sample collection at the Fundação SOS animal shelter; the owner of the Fundação SOS animal shelter, Ruth Marinho, who gave all of the structural and financial support to maintain the animals in good condition during captivity; the owner of the animal shelter in Vila Isabel, Maria José Maia de Miranda, for letting us work with samples from treated domestic cats; Mônica Barcellos Arruda and Ana Flávia Nacif Pinto Coelho Pires for laboratory technical support; Artur Trancoso Lopo de Queiroz for provide some scripts for ML analysis; Orlando C. Ferreira, Jr., for discussions of statistical analysis; and Davis F. Ferreira for gently revising the manuscript.
Footnotes
Published ahead of print on 11 June 2008.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Arai, M., D. D. Earl, and J. K. Yamamoto. 2002. Is AZT/3TC therapy effective against FIV infection or immunopathogenesis? Vet. Immunol. Immunopathol. 85189-204. [DOI] [PubMed] [Google Scholar]
- 2.Arion, D., N. Kaushik, S. McCormick, G. Borkow, and M. A. Parniak. 1998. Phenotypic mechanism of HIV-1 resistance to 3′-azido-3′-deoxythymidine (AZT): increased polymerization processivity and enhanced sensitivity to pyrophosphate of the mutant viral reverse transcriptase. Biochemistry 3715908-15917. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann, M. H., C. Mathiason-Dubard, G. H. Learn, A. G. Rodrigo, D. L. Sodora, P. Mazzetti, E. A. Hoover, and J. I. Mullins. 1997. Genetic diversity of feline immunodeficiency virus: dual infection, recombination, and distinct evolutionary rates among envelope sequence clades. J. Virol. 714241-4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boucher, C. A., E. O'Sullivan, J. W. Mulder, C. Ramautarsing, P. Kellam, G. Darby, J. M. Lange, J. Goudsmit, and B. A. Larder. 1992. Ordered appearance of zidovudine resistance mutations during treatment of 18 human immunodeficiency virus-positive subjects. J. Infect. Dis. 165105-110. [DOI] [PubMed] [Google Scholar]
- 5.Caldas, A. P. F., E. S. Leal, E. F. A. Silva, and A. P. Ravazzolo. 2000. Detecção do provirus da imunodeficiência felina em gatos domésticos pela técnica de reação em cadeia da polimerase. Pesq. Vet. Bras. 2020-25. [Google Scholar]
- 6.Carpenter, M. A., E. W. Brown, D. W. MacDonald, and S. J. O'Brien. 1998. Phylogeographic patterns of feline immunodeficiency virus genetic diversity in the domestic cat. Virology 251234-243. [DOI] [PubMed] [Google Scholar]
- 7.Caxito, F. A., F. M. Coelho, M. E. Oliveira, and M. Resende. 2006. Feline immunodeficiency virus subtype B in domestic cats in Minas Gerais, Brazil. Vet. Res. Commun. 30953-956. [DOI] [PubMed] [Google Scholar]
- 8.Greene, W. K., J. Meers, G. del Fierro, P. R. Carnegie, and W. F. Robinson. 1993. Extensive sequence variation of feline immunodeficiency virus env genes in isolates from naturally infected cats. Arch. Virol. 13351-62. [DOI] [PubMed] [Google Scholar]
- 9.Gu, Z., Q. Gao, H. Fang, H. Salomon, M. A. Parniak, E. Goldberg, J. Cameron, and M. A. Wainberg. 1994. Identification of a mutation at codon 65 in the IKKK motif of reverse transcriptase that encodes human immunodeficiency virus resistance to 2′,3′-dideoxycytidine and 2′,3′-dideoxy-3′-thiacytidine. Antimicrob. Agents Chemother. 38275-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrigan, P. R., C. Stone, P. Griffin, I. Nájera, S. Bloor, S. Kemp, M. Tisdale, and B. Larder. 2000. Resistance profile of the human immunodeficiency virus type 1 reverse transcriptase inhibitor abacavir (1592U89) after monotherapy and combination therapy. J. Infect. Dis. 181912-920. [DOI] [PubMed] [Google Scholar]
- 11.Hayward, J. J., J. Taylor, and A. G. Rodrigo. 2007. Phylogenetic analysis of feline immunodeficiency virus in feral and companion domestic cats of New Zealand. J. Virol. 812999-3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hohdatsu, T., H. Hirabayashi, K. Motokawa, and H. Koyama. 1996. Comparative study of the cell tropism of feline immunodeficiency virus isolates of subtypes A, B and D classified on the basis of the env gene V3-V5 sequence. J. Gen. Virol. 7793-100. [DOI] [PubMed] [Google Scholar]
- 13.Imamichi, T., S. C. Berg, H. Imamichi, J. C. Lopez, J. A. Metcalf, J. Falloon, and H. C. Lane. 2000. Relative replication fitness of a high-level 3′-azido-3′-deoxythymidine-resistant variant of human immunodeficiency virus type 1 possessing an amino acid deletion at codon 67 and a novel substitution (Thr→Gly) at codon 69. J. Virol. 7410958-10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imamichi, T., T. Sinha, H. Imamichi, Y.-M. Zhang, J. A. Metcalf, J. Falloon, and H. C. Lane. 2000. High-level resistance to 3′-azido-3′-deoxythimidine due to a deletion in the reverse transcriptase gene of human immunodeficiency virus type 1. J. Virol. 741023-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inada, G., T. Miyazawa, Y. Inoshima, M. Kohmoto, Y. Ikeda, C. H. Liu, J. A. Lin, T. F. Kuo, and T. Mikami. 1997. Phylogenetic analysis of feline immunodeficiency virus isolated from cats in Taiwan. Arch. Virol. 1421459-1467. [PubMed] [Google Scholar]
- 16.Ishida, T., T. Washizu, K. Toriyabe, S. Motoyoshi, I. Tomoda, and N. C. Pedersen. 1989. Feline immunodeficiency virus infection in cats of Japan. J. Am. Vet. Med. Assoc. 194221-225. [PubMed] [Google Scholar]
- 17.Kakinuma, S., K. Motokawa, T. Hohdatsu, J. K. Yamamoto, H. Koyama, and H. Hashimoto. 1995. Nucleotide sequence of feline immunodeficiency virus: classification of Japanese isolates into two subtypes which are distinct from non-Japanese subtypes. J. Virol. 693639-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA 3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5150-163. [DOI] [PubMed] [Google Scholar]
- 19.Kuritzkes, D. R. 2007. HIV resistance: frequency, testing, mechanisms. Top. HIV Med. 15150-154. [PubMed] [Google Scholar]
- 20.Lole, K. S., R. C. Bollinger, R. S. Paranjape, D. Gadkari, S. S. Kulkarni, N. G. Novak, R. Ingersoll, H. W. Sheppard, and S. C. Ray. 1999. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 73152-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Margot, N. A., E. Isaacson, I. McGowan, A. K. Cheng, R. T. Schooley, and M. D. Miller. 2002. Genotypic and phenotypic analyses of HIV-1 in antiretroviral-experienced patients treated with tenofovir. AIDS 161227-1235. [DOI] [PubMed] [Google Scholar]
- 22.Margot, N. A., E. Isaacson, I. McGowan, A. Cheng, and M. D. Miller. 2003. Extended treatment with tenofovir disoproxil fumarate in treatment-experienced HIV-1-infected patients: genotypic, phenotypic, and rebound analyses. J. Acquir. Immune Defic. Syndr. 3315-21. [DOI] [PubMed] [Google Scholar]
- 23.Masciari, R., L. Cosco, M. C. Diaco, D. N. Della, T. Ferraro, T. Raimondi, B. Ruperti, and E. Santandrea. 2002. HIV-1: a case of RT67 deletion in a multi-treated non responder patient. New Microbiol. 2583-88. [PubMed] [Google Scholar]
- 24.Medlin, H. K., Y.-Q. Zhu, K. M. Remington, T. R. Phillips, and T. W. North. 1996. Selection and characterization of a mutant of feline immunodeficiency virus resistant to 2′,3′-dideoxycytidine. Antimicrob. Agents Chemother. 40953-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller, V., M. Ait-Khaled, C. Stone, P. Griffin, D. Mesogiti, A. Cutrell, R. Harrigan, S. Staszewski, C. Katlama, G. Pearce, and M. Tisdale. 2000. HIV-1 reverse transcriptase (RT) genotype and susceptibility to RT inhibitors during abacavir monotherapy and combination therapy. AIDS 14163-171. [DOI] [PubMed] [Google Scholar]
- 26.Moraillon, A. 1990. Feline immunodepressive retrovirus infections in France. Vet. Rec. 12668-69. [PubMed] [Google Scholar]
- 27.Nakamura, K., Y. Suzuki, K. Ikeo, Y. Ikeda, E. Sato, N. T. P. Nguyen, T. Gojobori, T. Mikami, and T. Miyazawa. 2003. Phylogenetic analysis of Vietnamese isolates of feline immunodeficiency virus: genetic diversity of subtype C. Arch. Virol. 148783-791. [DOI] [PubMed] [Google Scholar]
- 28.Pecoraro, M. R., K. Tomonaga, T. Miyazawa, Y. Kawaguchi, S. Sugita, Y. Tohya, C. Kai, M. E. Etcheverrigaray, and T. Mikami. 1996. Genetic diversity of Argentine isolates of feline immunodeficiency virus. J. Gen. Virol. 772031-2035. [DOI] [PubMed] [Google Scholar]
- 29.Pistello, M., G. Cammarota, E. Nicoletti, D. Matteucci, M. Curcio, D. Del Mauro, and M. Bendinelli. 1997. Analysis of the genetic diversity and phylogenetic relationship of Italian isolates of feline immunodeficiency virus indicates a high prevalence and heterogeneity of subtype B. J. Gen. Virol. 782247-2257. [DOI] [PubMed] [Google Scholar]
- 30.Posada, D., and K. A. Crandall. 1998. MODELTEST: testing the model of DNA substitution. Bioinformatics 14817-818. [DOI] [PubMed] [Google Scholar]
- 31.Poss, M., H. A. Ross, S. L. Painter, D. C. Holley, J. A. Terwee, S. VandeWoude, and A. Rodrigo. 2006. Feline lentivirus evolution in cross-species infection reveals extensive G-to-A mutation and selection on key residues in the viral polymerase. J. Virol. 802728-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reche, A., Jr., M. K. Hagiwara, and S. R. R. Lucas. 1999. Estudo clínico da síndrome de imunodeficiência adquirida em gatos domésticos de São Paulo. Braz. J. Vet. Res. Anim. Sci. 34152-155. [Google Scholar]
- 33.Reggeti, F., and D. Bienzle. 2004. Feline immunodeficiency virus subtypes A, B and C and intersubtype recombinants in Ontario, Canada. J. Gen. Virol. 851843-1852. [DOI] [PubMed] [Google Scholar]
- 34.Rigby, M. A., E. C. Holmes, M. Pistello, A. Mackay, A. J. Brown, and J. C. Neil. 1993. Evolution of structural proteins of feline immunodeficiency virus: molecular epidemiology and evidence of selection for change. J. Gen. Virol. 74425-436. [DOI] [PubMed] [Google Scholar]
- 35.Ross, L., M. Johnson, R. G. Ferris, S. A. Short, L. R. Boone, T. E. Melby, R. Lanier, M. Shaefer, and M. St Clair. 2000. Deletions in the beta3-beta4 hairpin loop of HIV-1 reverse transcriptase are observed in HIV-1 isolated from subjects during long-term antiretroviral therapy. J. Hum. Virol. 3144-149. [PubMed] [Google Scholar]
- 36.Savarino, A., M. Pistello, D. D'Ostilio, E. Zabogli, F. Taglia, F. Mancini, S. Ferro, D. Matteucci, L. De Luca, M. L. Barreca, A. Ciervo, A. Chimirri, M. Ciccozzi, and M. Bendinelli. 2007. Human immunodeficiency virus integrase inhibitors efficiently suppress feline immunodeficiency virus replication in vitro and provide a rationale to redesign antiretroviral treatment for feline AIDS. Retrovirology 479. doi: 10.1186/1742-4690-4-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith, R. A., K. M. Remington, R. M. Lloyd, Jr., R. F. Schinazi, and T. W. North. 1997. A novel Met-to-Thr mutation in the YMDD motif of reverse transcriptase from feline immunodeficiency virus confers resistance to oxathiolane nucleosides. J. Virol. 712357-2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith, R. A., K. M. Remington, B. D. Preston, R. F. Schinazi, and T. W. North. 1998. A novel point mutation at position 156 of reverse transcriptase from feline immunodeficiency virus confers resistance to the combination of (−)-β-2′,3′-dideoxy-3′-thiacytidine and 3′-azido-3′-deoxythymidine. J. Virol. 722335-2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sodora, D. L., E. G. Shpaer, B. E. Kitchell, S. W. Dow, E. A. Hoover, and J. I. Mullins. 1994. Identification of three feline immunodeficiency virus (FIV) env gene subtypes and comparison of the FIV and human immunodeficiency virus type 1 evolutionary patterns. J. Virol. 682230-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Souza, H. J. M., C. H. Teixeira, and R. Graça. 2002. Estudo epidemiológico de infecções pelo vírus da leucemia e/ou imunodeficiência felina, em gatos domésticos do município do Rio de Janeiro. Clín. Vet. 3614-21. [Google Scholar]
- 41.Steinrigl, A., and D. Klein. 2003. Phylogenetic analysis of feline immunodeficiency virus in Central Europe: a prerequisite for vaccination and molecular diagnostic. J. Gen. Virol. 841301-1307. [DOI] [PubMed] [Google Scholar]
- 42.Swofford, D. L., G. J. Olsen, P. J. Waddell, and D. M. Hillis. 1996. Phylogenetic inference, p. 407-514. In D. M. Hillis, C. Moritz, and B. K. Mable (ed.), Molecular systematics. Sinauer, Sunderland, MA.
- 43.Swofford, D. L. 2003. PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. Sinauer Associates, Sunderland, MA.
- 44.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 224673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uhl, E. W., T. G. Heaton-Jones, R. Pu, and J. K. Yamamoto. 2002. FIV vaccine development and its importance to veterinary and human medicine: a review FIV vaccine 2002 update and review. Vet. Immunol. Immunopathol. 90113-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winston, A., S. Mandalia, D. Pillay, B. Gazzard, and A. Pozniak. 2002. The prevalence and determinants of the K65R mutation in HIV-1 reverse transcriptase in tenofovir-naive patients. AIDS 162087-2089. [DOI] [PubMed] [Google Scholar]
- 47.Winters, M. A., R. W. Shafer, R. A. Jellinger, G. Mamtora, T. Gingeras, and T. C. Merigan. 1997. Human immunodeficiency virus type 1 reverse transcriptase genotype and drug susceptibility changes in infected individuals receiving dideoxyinosine monotherapy for 1 to 2 years. Antimicrob. Agents Chemother. 41757-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winters, M. A., K. L. Coolley, P. Cheng, Y. A. Girard, H. Hamdan, L. C. Kovari, and T. C. Merigan. 2000. Genotypic, phenotypic, and modeling studies of a deletion in the β3-β4 region of the human immunodeficiency virus type 1 reverse transcriptase gene that is associated with resistance to nucleoside reverse transcriptase inhibitors. J. Virol. 7410707-10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamamoto, J. K., R. Pu, E. Sato, and T. Hohdatsu. 2007. Feline immunodeficiency virus pathogenesis and development of a dual-subtype feline-immunodeficiency-virus vaccine. AIDS 21547-563. [DOI] [PubMed] [Google Scholar]
- 50.Zhang, D., A. M. Caliendo, J. J. Eron, K. M. DeVore, J. C. Kaplan, M. S. Hirsch, and R. T. D'Aquila. 1994. Resistance to 2′,3′-dideoxycytidine conferred by a mutation in codon 65 of the human immunodeficiency virus type 1 reverse transcriptase. Antimicrob. Agents Chemother. 38282-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu, Y. Q., K. M. Remington, and T. W. North. 1996. Mutants of feline immunodeficiency virus resistant to 2′,3′-dideoxy-2′,3′-didehydrothymidine. Antimicrob. Agents Chemother. 401983-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.