Abstract
Human immunodeficiency virus type 1 (HIV-1) preferentially utilizes the CCR5 coreceptor for target cell entry in the acute phase of infection, while later in disease progression the virus switches to the CXCR4 coreceptor in approximately 50% of patients. In response to HIV-1 the adaptive immune response is triggered, and antibody (Ab) production is elicited to block HIV-1 entry. We recently determined that dendritic cells (DCs) can efficiently capture Ab-neutralized HIV-1, restore infectivity, and transmit infectious virus to target cells. Here, we tested the effect of Abs on trans transmission of CCR5 or CXCR4 HIV-1 variants. We observed that transmission of HIV-1 by immature as well as mature DCs was significantly higher for CXCR4- than CCR5-tropic viral strains. Additionally, neutralizing Abs directed against either the gp41 or gp120 region of the envelope such as 2F5, 4E10, and V3-directed Abs inhibited transmission of CCR5-tropic HIV-1, whereas Ab-treated CXCR4-tropic virus demonstrated unaltered or increased transmission. To further study the effects of coreceptor usage we tested molecularly cloned HIV-1 variants with modifications in the envelope that were based on longitudinal gp120 V1 and V3 variable loop sequences from a patient progressing to AIDS. We observed that DCs preferentially facilitated infection of CD4+ T lymphocytes of viral strains with an envelope phenotype found late in disease. Taken together, our results illustrate that DCs transmit CXCR4-tropic HIV-1 much more efficiently than CCR5 strains; we hypothesize that this discrimination could contribute to the in vivo coreceptor switch after seroconversion and could be responsible for the increase in viral load.
Human immunodeficiency virus type 1 (HIV-1) primarily infects CD4+ T lymphocytes of the immune system with monocytes, macrophages, Langerhans cells (LCs), and dendritic cells (DCs) also susceptible to infection (31, 39, 45, 53). For HIV-1 entry into target cells, the viral envelope must first engage with the CD4 receptor, followed by interaction with a chemokine coreceptor, the two most prominent being CCR5 and CXCR4. Viruses utilizing CCR5 (designated R5 variants) are found predominantly at time of transmission and early in infection, with the CXCR4-using viruses (designated X4 variants) found later in disease in 50% of patients (3). The factors determining this bottleneck in R5 transmission and the subsequent emergence of X4 variants is unknown, although the type of cell infected and better immune control of X4 viruses early in disease have been suggested and critically reviewed (5, 38, 51).
It has been shown that LCs are among the first cells HIV-1 encounters in the mucosal epithelia (18, 25, 26, 47). Although LCs can be infected with HIV-1 at high viral input, the majority of virus is captured by the cell protein langerin and degraded in Birbeck granules after internalization (9, 33, 37). In the subepithelium HIV-1 encounters immature DCs (iDCs) which do not express langerin but express other C-type lectins, such as DC-SIGN (DC-specific ICAM-3 grabbing nonintegrin), that capture HIV-1 by interacting with the viral gp120 envelope protein (12). Although most captured virus is degraded by iDCs, a fraction can be transmitted to CD4+ T lymphocytes in trans. Transmission of virus occurs via the formation of an immunological synapse in which HIV-1 is recruited to the contact site between the DC and CD4+ T lymphocyte (1). At this site CD4 and coreceptors are concentrated on the lymphocyte membrane, leading to efficient HIV-1 trans infection of CD4+ T lymphocytes (11, 24). DCs can also be infected with HIV-1. Transmission of de novo produced virions to CD4+ T lymphocytes by DCs occurs approximately after 48 h and is termed transmission in cis (7, 8, 10, 44, 49). Although iDCs carry the CCR5 as well as CXCR4 coreceptor, only R5 viruses are efficiently produced by this cell type, which may partially explain the preferential outgrowth of R5 variants upon horizontal sexual transmission. It has been postulated that productive infection of DCs or LCs followed by HIV-1 transmission in cis is responsible for the onset of acute infection rather than transmission in trans (17, 47). Upon infection or capture of HIV-1 by iDCs, the cells differentiate into mature DCs (13). Since mature DCs leave the epithelial layer and migrate to lymph nodes, DCs are thought to act as a Trojan horse to deliver HIV-1 to a pool of susceptible CD4+ T lymphocytes (36).
Infection with HIV-1 induces an adaptive immune response in the host, leading to the production of antibodies (Abs). Neutralizing Abs (NAbs) bind to the viral envelope and prevent infection while nonneutralizing Abs can mediate their effects via either the induction of antibody-dependent cell-mediated cytotoxicity responses (14) or via complement-mediated virion lysis (19). The nonneutralizing Abs induced in the early acute phase of infection have been associated with control of viral load via complement virion lysis, while NAbs appear later in disease. Moreover, both types of Ab inhibit HIV-1 replication in iDCs, thereby preventing transmission of de novo produced HIV-1 (15, 16). Despite the high variation in the Ab repertoire only a few broadly NAbs directed against the gp41 region, Abs 2F5 and 4E10 (27, 34, 35, 46, 55), or the gp120 envelope region, Abs 2G12 and b12 (2, 42), have been found to efficiently block HIV-1 target cell infection. Passive immunization of rhesus macaques with these NAbs partially protected the animals from long-term infection (2, 43), whereas immunization of acutely or chronically infected HIV-1 patients with a cocktail of 2F5, 4E10, and 2G12 Abs decreased viral load temporarily (48). Interestingly, these patients developed 2G12-sensitive viral escape mutants, whereas no escape mutants were observed against the 2F5 or 4E10 Abs (22), suggesting that HIV-1 can efficiently circumvent neutralization via another mechanism (52).
We have previously demonstrated that HIV-1 neutralized with either 2F5, 4E10, or other broadly NAbs can be efficiently captured by immature monocyte-derived dendritic cells (iMDDCs) and transferred to CD4+ T lymphocytes (50), in a process we called trans-transmission. Here, we investigated whether capture and transfer by iMDDCs is influenced by the viral phenotype. We show that iMDDCs as well as Raji cells expressing DC-SIGN (Raji-DC-SIGN) capture and transfer preferentially X4 opposed to R5 variants. We demonstrate that NAb-treated R5 HIV-1 is always more strongly inhibited in transmission by iMDDCs or Raji-DC-SIGN cells than NAb-treated X4 HIV-1. Furthermore, we demonstrate that other types of DCs preferentially transmit X4 HIV-1 over R5 strains in the presence or absence of NAbs. Taken together, our data suggest that in the presence of Abs the more efficient transmission of X4 HIV-1 by DCs may play a role in the often observed switch from R5 to X4 during disease progression after initiation of the adaptive immune response.
MATERIALS AND METHODS
Antibodies and reagents.
The DC-SIGN-specific monoclonal Ab (MAb) AZN-D1 was used to block interaction of HIV-1 with DC-SIGN; an immunoglobulin G1 (IgG1) mouse MAb (ITK Diagnostics) was included as an isotype control. Human MAbs 2F5 and 4E10 directed against HIV-1 envelope gp41; Abs 2G12, 1.7b, 4.8D, and b12 directed against gp120; and Abs V3-13 and V3-21 directed against the V3 loop of gp120 were obtained from the National Institute for Biological Standards and Control. All Abs had the IgG1 isotype and were used at 20 μg/ml. Soluble CD4 (sCD4) (20 μg/ml) and indinavir (1 μM) were obtained from National Institute for Biological Standards and Control. Phycoerythrin (PE)-labeled DC-SIGN, CD3-labeled allophycocyanin, CD1A-fluorescein isothiocyanate (FITC), CD14-FITC, CD83-PE, CD86-FITC, HLA-DR-peridinin chlorophyll protein, CD11b, CD11c, streptavidin-peridinin chlorophyll protein-Cy5.5 (BD-Pharmingen), biotinylated ICAM-1 (R&D Systems, Abingdon, United Kingdom), donkey anti-human-Cy5 (Jackson ImmunoResearch Europe Ltd., Suffolk, United Kingdom), and CA-p24 FITC were utilized for fluorescence-activated cell sorting (FACS) analyses at a 50- or 200-fold dilution for primary and secondary Abs, respectively.
Cells.
The Raji control cell line and Raji-DC-SIGN cells were generated and used as described previously (12). Both cell lines were cultured in RPMI 1640 medium containing 10% fetal calf serum. DC-SIGN expression by Raji-DC-SIGN cells was positively selected with neomycin (2 μg/ml) and routinely monitored by FACS analysis using the PE-labeled DC-SIGN Ab. The iMDDCs were prepared as previously described (40). In short, human blood monocytes were isolated from buffy coats by use of a Ficoll gradient and a subsequent CD14 selection step using a magnetic bead cell sorting system (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). Purified monocytes were differentiated into iMDDCs in the presence of interleukin-4 (IL-4) and granulocyte-macrophage colony-stimulating factor (500 and 800 U/ml, respectively; Schering-Plough, Brussels, Belgium) and used on day 6. Mature monocyte-derived DCs (mMDDCs) were obtained on day 6 after stimulating iMDDCs on day 5 with 20 μg of poly(I·C) per ml (Sigma-Aldrich, St. Louis, MO). The phenotypes of both types of DCs were confirmed by flow cytometry with major histocompatibility complex class II (MHC-II) molecules, CD1a, CD11b, CD11c, CD14, ICAM-1, CD83, and CD86. Low-level surface expression of CD83, CD86, and MHC-II was detected in iMDDCs with high DC-SIGN expression, whereas mMDDCs positively stained with the CD83, CD86, and MHC-II Abs with a reduced DC-SIGN expression (data not shown). Myeloid DCs (mDCs) were isolated from PBMCs with a BDCA-1+ DC isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). In short, CD19+ B cells were removed from peripheral blood mononuclear cells (PBMCs), Fc receptors were blocked, and mDCs were isolated by positive selection with the BDCA-1-biotin and antibiotin microbeads. PBMCs were isolated from fresh buffy coats (Central Laboratory Blood Bank, Amsterdam, The Netherlands) by standard Ficoll-Hypaque density centrifugation and checked by PCR for the CCR5 Δ32 allele. PBMCs from three donors were pooled, frozen in multiple vials, and, when required, thawed, activated with phytohemagglutinin at 2 μg/ml and cultured in RPMI medium supplemented with recombinant IL-2 (rIL-2) at 100 U/ml. On day 3 of culture, CD4+ T lymphocytes were enriched by depleting CD8+ T lymphocytes using CD8 immunomagnetic beads (Dynal, Invitrogen, Breda, The Netherlands); CD4+ T lymphocytes were cultured for 2 days in RPMI medium with rIL-2.
Virus.
C33A cervix carcinoma cells were transfected with 40 ng of JR-CSF (R5), LAI (X4), or envelope-modified molecular cloned HIV-1 proviral DNA per 75-cm2 flask. Molecularly cloned HIV-1 constructs contain the LAI backbone with an HXB2 envelope. Mutations in the V1 and V3 variable loops of the HXB2 envelope were generated according to mutations identified in the envelope from a patient from the Amsterdam cohort studies (ACH168) (32). Virus stock was assayed for the 50% tissue culture infectious dose (TCID50) on CD4+ enriched T lymphocytes.
Virus capture.
Neutralized or control HIV-1 (CA-p24 at 15 to 40 ng/ml) was incubated for 2 h at 37°C with 4.0 × 106 Raji or Raji-DC-SIGN cells. Unbound virus was removed by washing three times with phosphate-buffered saline (PBS) or medium. Cells were lysed in 1% empigen and incubated at 56°C for 1 h. Cell debris was pelleted, and HIV-1 capture was determined by CA-p24 detection with enzyme-linked immunosorbent assay (ELISA) in quadruplicates.
Virus neutralization.
A fixed dose of HIV-1, 200 TCID50s for infection and 400 TCID50s for transmission, was incubated for 1 h at 37°C with NAb or control IgG1 Ab before experimental use.
HIV-1 infection.
Phytohemagglutinin-activated CD4+ T lymphocytes (1.5 × 105/well) were cocultured with or without Raji, Raji-DC-SIGN, or iMDDCs (0.3 × 105/well) untreated or treated with Ab AZN-D1 for 30 min and inoculated with 200 to 5,000 TCID50s of control or neutralized HIV-1 in the continued presence of control Ab or NAb. Medium was removed after 48 h, and cells were cultured in fresh RPMI medium containing rIL-2 (100 U/ml) and indinavir (1 μM) for 3 days. Infection of CD4+ T lymphocytes was measured by following intracellular CA-p24 expression by FACS flow cytometry per 1.0 × 105 CD3+ T lymphocytes.
HIV-1 transmission.
A total of 1.0 × 105 iMDDCs, mMDDCs, mDCs, or Raji-DC-SIGN cells were incubated for 2 h with neutralized or control HIV-1 at 37°C. Unbound virus was removed by washing, and cells were cocultured with 1.5 × 105 CD4+ T lymphocytes in a 96-well plate. Medium was removed after 48 h, and cells were cultured in fresh RPMI medium containing rIL-2 (2 μg/ml) and indinavir (1 μM) for 3 days. Transmission was determined as the number of infected CD4+ T lymphocytes measured by following intracellular CA-p24 expression by FACS flow cytometry per 1.0 × 105 CD3+ T lymphocytes.
FACS staining.
Cells were washed three times with cold PBS and fixed in 3.7% paraformaldehyde in PBS for 20 min. Fixative was quenched with 20 mM glycine in PBS for 10 min, and cells were permeabilized in 0.1% saponin, 1% bovine serum albumin in PBS for 30 min and subsequently stained with FITC-labeled CA-p24 and CD3-labeled allophycocyanin for 1 h. Excess Ab was removed by two washes with permeabilization buffer and one wash with 1% bovine serum albumin in PBS. Subsequently, cells were resuspended and maintained in PBS until analyzed.
Statistics.
Statistical significance was determined using an unpaired t test (two-tailed) and indicated with stars.
RESULTS
Raji-DC-SIGN cells mediate efficient transmission of 2F5-neutralized X4 but not R5 virus.
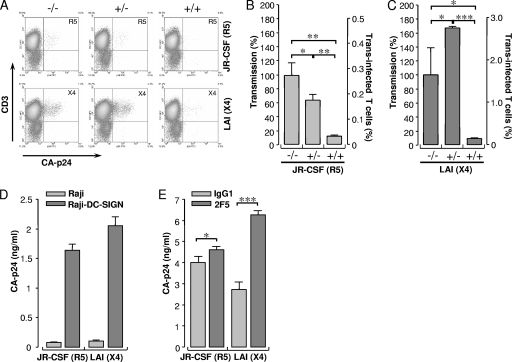
We previously demonstrated that an R5X4 strain of HIV-1 preincubated with a fully neutralizing concentration of the 2F5 Ab was more efficiently transmitted to CD4+ T lymphocytes than nonneutralized virus (50). Here, we investigated the transmission of JR-CSF (R5) or LAI (X4) by Raji-DC-SIGN cells when virus was pretreated with 2F5 Ab. Raji-DC-SIGN cells were loaded either with control- or 2F5-treated R5 or X4 virus. After removal of unbound virus, cells were incubated with CD4-enriched T lymphocytes to quantify transmission by measuring CA-p24 trans infected CD4+ T lymphocytes by FACS flow cytometry (Fig. 1A). The transmission efficiency of 2F5-neutralized R5 HIV-1 by Raji-DC-SIGN cells was 62% (P < 0.05) compared to transmission of control Ab-treated R5 HIV-1 (Fig. 1B). Transmission could be blocked to a residual 10% (P < 0.005) by readdition of fresh 2F5 Ab, implying that captured 2F5-neutralized R5 HIV-1 was released as infectious virus. In contrast to the R5 virus strain, transmission of the 2F5-neutralized X4 variant by Raji-DC-SIGN cells increased to 160% (P < 0.05) compared to the isotype control (Fig. 1C). This indicates that the X4 HIV-1 variant has an advantage over the R5 strain in transmission when neutralized with the 2F5 Ab. Although transmission was enhanced for 2F5-neutralized X4 HIV-1, readdition of 2F5 efficiently blocked transmission by 90% (P < 0.0001), similar to results with the R5 variant, illustrating that both strains were sensitive to 2F5 neutralization upon transmission.
FIG. 1.
Raji-DC-SIGN cells discriminate between transmission of 2F5-neutralized X4 and R5 HIV-1. (A) Transmission of control R5 (upper row) or X4 (lower row) (−/−) or 2F5-treated R5 or X4 (−/+) by Raji-DC-SIGN cells and transmission of 2F5-treated virus in the presence of 2F5 NAb in the subsequent culture with CD4-cells (+/+). The percentage of transmission was normalized to control virus and plotted as a percentage of trans infected CD4+ T lymphocytes. (B) Transmission of R5 HIV-1. (C) Transmission of X4 HIV-1. Data are shown as mean values of triplicates ± standard deviations (error bars). (D) Capture of R5 or X4 HIV-1 by Raji or Raji-DC-SIGN cells with 15 ng of CA-p24 viral input per ml. (E) Capture of 2F5-neutralized R5 or X4 HIV-1 by Raji-DC-SIGN cells with a 40 ng of CA-p24 viral input per ml. Results represent data from three independent experiments. *, P ≤ 0.05; **, P ≤ 0.005; ***, P ≤ 0.0005.
To investigate whether the differences observed in transmission of 2F5-neutralized R5 or X4 HIV-1 were mediated by differences in viral binding to DC-SIGN, we measured intracellular viral CA-p24 captured by Raji-DC-SIGN or control Raji cells by ELISA (Fig. 1D). R5 (1.6 ± 0.1 CA-p24 ng/ml) and X4 HIV-1 (2.1 ± 0.2 CA-p24 ng/ml) were both efficiently captured by Raji-DC-SIGN cells, whereas DC-SIGN-negative cells did not capture either variant. These results indicate that the difference observed in transmission of R5 and X4 HIV-1 was not caused by variation in capture. Neutralization of R5 HIV-1 with 2F5 Ab had a limited effect on capture, whereas X4 HIV-1 capture was increased twofold (P < 0.0001) upon 2F5-neutralization (Fig. 1E). These results suggest that the increase in transmission observed in Fig. 1C is probably mediated by an increase in capture of neutralized X4 HIV-1. Interestingly, although 2F5 neutralization of R5 virus had only a moderate positive effect on DC-SIGN-mediated capture, a decrease in transmission was observed (Fig. 1B), suggesting that a proportion of transmitted R5 virus remained neutralized during transmission.
Effect of variant Abs on HIV-1 transfer.
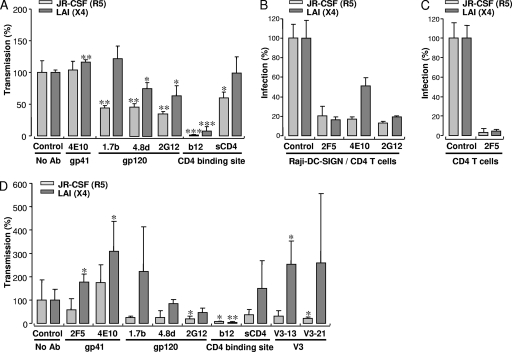
Since the 2F5 Ab had a differential transmission effect on R5 and X4 viruses, we tested additional Abs, one directed against gp41 and four against gp120 (Fig. 2A). Neutralization of R5 HIV-1 with the gp41-directed Ab 4E10 did not alter transmission by Raji-DC-SIGN cells, whereas a small increase was observed for the X4 LAI virus (116%, P < 0.005). Preneutralization of JR-CSF with 2G12 prior to capture efficiently blocked transmission to 36%, whereas transmission of LAI was blocked to 63%. The CD4-dependent Abs 1.7b and 4.8d were capable of inhibiting transmission of the R5 virus to 44% and 46%, respectively, whereas transmission of 4.8d-treated X4 virus was reduced to 75%; no effect was measured with the 1.7b Ab. These results with the 1.7b and 4.8d Abs indicate that HIV-1 binding occurs under the assay conditions used. The only Ab that efficiently blocked transmission of both virus strains was b12. These data imply that many Abs lose the capacity to neutralize HIV-1 during capture and transmission by Raji-DC-SIGN cells and that X4 HIV-1 is always more efficiently transmitted after treatment with Abs before capture than R5 virus. Since the b12 Ab targets the CD4-binding site of the envelope, we tested the effect of sCD4 on transmission of R5 and X4 HIV-1 variants. Transmission of sCD4-treated JR-CSF was reduced to 55%, whereas no effect was observed for the LAI strain treated with sCD4. These results indicate that Raji-DC-SIGN cells can capture X4 as well as R5 HIV-1 in complex with sCD4 and that subsequent transmission is possible.
FIG. 2.
Effect of different antibodies and sCD4 on transmission and infection. (A) Transmission by Raji-DC-SIGN cells of R5 and X4 HIV-1 pretreated with the Abs 4E10, 1.7b, 4.8d, 2G12, b12, or with sCD4. Transmission rates of R5- and X4-treated HIV-1 were normalized for transmission of control virus and plotted as mean values of triplicates ± standard deviations. (B) Infection of CD4+ cells with neutralized or untreated R5 and X4 HIV-1 (200 TCID50s) in the presence of Raji-DC-SIGN cells. (C) Infection of untreated or 2F5-neutralized R5 and X4 HIV-1 (5,000 TCID50s). Data represent the results of two independent experiments and are plotted as mean values of quadruplicates ± standard deviations, normalized to control HIV-1. (D) Transmission of R5 and X4 HIV-1 treated with gp41-directed Abs 2F5 and 4E10; gp120-directed Abs 1.7b, 4.8d, 2G12, and b12; V3-directed Abs V3-13 and V3-21; or sCD4 by iMDDCs. Data are plotted as mean values of quadruplicates ± standard deviations, normalized to control HIV-1, and represent the results of three independent experiments. *, P ≤ 0.05; **, P ≤ 0.005; ***, P ≤ 0.0005.
We next determined the Ab neutralization sensitivity of both the R5 and X4 strains with 2F5, 4E10, and 2G12 on CD4+ T lymphocytes in the presence of Raji-DC-SIGN cells. Both viruses were efficiently neutralized by ∼80% with the 2F5 and 2G12 Abs (Fig. 2B). In contrast, the 4E10 Ab was less efficient at neutralizing LAI (∼50%) than JR-CSF (∼83%). To determine the 2F5 neutralization sensitivity of JR-CSF and LAI in the absence of Raji-DC-SIGN cells, we increased the viral input 25-fold to 5,000 TCID50s to measure direct infection of CD4+ T lymphocytes (Fig. 2C). Infection of both strains was fully inhibited in the presence of 2F5. Together, these results indicate that the difference in transmission efficiency of neutralized R5 and X4 viruses is not caused by variations in neutralization sensitivity.
Since the above results were obtained with Raji-DC-SIGN cells (12), we studied the effect of Abs on transmission of R5 and X4 viruses by iMDDCs derived from human blood monocytes (Fig. 2D). Here we included Abs directed against the V3 region of the envelope (Abs V3-13 and V3-21). Transmission of 2F5-treated JR-CSF was reduced to 63% of control-treated virus, whereas transmission of 2F5-treated LAI was increased to 170% (P < 0.05), similar to results previously observed with Raji-DC-SIGN cells (Fig. 1C). Transmission of LAI was increased upon treatment with 4E10 Ab (P < 0.05), whereas treatment had no effect on JR-CSF. Transmission of R5 and X4 viruses by iMDDCs with respect to 1.7b, 4.8d, 2G12, or b12 Ab neutralization was highly comparable to transmission by Raji-DC-SIGN cells. Moreover, both V3-directed Abs, V3-13 and V3-21, stimulated transmission of LAI and blocked transmission of JR-CSF, suggesting that X4 viruses have a strong beneficial transmission advantage over R5 viruses when neutralized with either gp41-directed Abs or V3-directed Abs.
Neutralized HIV-1 can be rendered infectious through capture by mMDDCs.
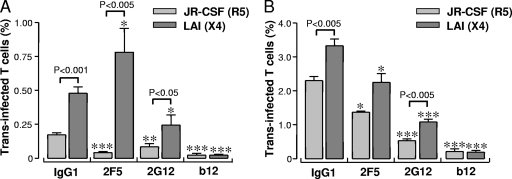
In terms of absolute viral transmission by iMDDCs measured as the number of infected CD4+ T lymphocytes, X4 HIV-1 is 2.4-fold (P < 0.001) more efficiently transferred than R5 virus (Fig. 3A). In the presence of the 2F5 Ab, the difference in transmission levels between the R5 and X4 viruses increased to 7.5-fold (Fig. 3A). A 2.2-fold difference in transmission of R5 and X4 HIV-1 was observed upon treatment with the 2G12 Ab, which partially blocked transfer of both viruses, and the b12 Ab fully blocked transmission of both strains.
FIG. 3.
mMDDCs transmit HIV-1 more efficiently than iMDDCs and facilitate transmission of X4 HIV-1 over R5 HIV-1. Transmission of R5 and X4 HIV-1 preneutralized with 2F5, 2G12, and b12 by iMDDCs and mMDDCs. Data represent the percentage of trans infected CD4+ T lymphocytes plotted as mean values of quadruplicates ± standard errors of the means of two independent experiments. *, P ≤ 0.05; **, P ≤ 0.005; ***, P ≤ 0.0005.
Next, we studied whether mMDDCs transferred R5 and X4 viruses at levels similar to iMDDCs. The mMDDCs transferred virus more efficiently to CD4+ T lymphocytes than did the iMDDCs cells (Fig. 3B), with increases of 10-fold for R5 and 7-fold for X4 HIV-1, which is in line with previous studies (41). As observed with iMDDCs, isolated mMDDCs transferred X4 virus more efficiently to CD4+ T lymphocytes than R5 virus (Fig. 3B). Treatment of HIV-1 with 2F5 or 2G12 reduced transmission of X4 virus (30% and 68%, respectively) and R5 virus (25% and 77%, respectively), whereas the b12 Ab fully blocked transmission of both strains. These results indicate that mMDDCs can also reverse 2F5 viral neutralization after capture.
mDCs preferentially transmit X4 HIV-1.
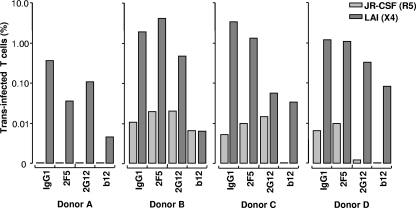
Since iMDDCs and mMDDCs are differentiated in vitro from monocytes, we characterized virus transfer with physiologically more relevant in vivo obtained mDCs. Isolated mDCs from four different donors (A to D) were analyzed by FACS cytometry, with ∼85% of the cells positive for BDCA-1 and CD11c while negative for CD3, 14, 16, 19, 20, 56 (data not shown). Transmission of R5 HIV-1 by mDCs was extremely low for all donors, with donor A demonstrating no viral transfer (Fig. 4). Interestingly, donor A was heterozygous for the CCR5 Δ32 allele, suggesting that CCR5 expression by mDCs may play a role in transmission of R5 HIV-1 in trans. All four donor mDCs showed a higher level of viral transfer with the X4 isolate than with the R5 strain. Treatment with 2F5 did not influence transmission of either virus strain, illustrating that mDCs can render neutralized virus particles infectious. Pretreatment of virus with 2G12 inhibited mDC transfer of JR-CSF from donor D by 81%, with LAI transfer inhibited by 73% for donors A, B, and D and 99% for donor C. As previously shown for the iMDDCs and mMDDCs, b12 strongly blocked transmission of both strains. Furthermore, these results demonstrate that mDCs can also transfer X4 HIV-1 more efficiently to susceptible CD4+ T lymphocytes whether the virus is neutralized or not.
FIG. 4.
mDCs preferentially transmit X4 HIV-1 over R5 HIV-1. R5 and X4 HIV-1 viruses were preincubated with 2F5, 2G12, or b12 Ab before capture by mDCs isolated from blood of four different donors, and transmission was measured as a percentage of trans infected CD4+ T lymphocytes and plotted in log scale.
Influence of the V1 and V3 loops of gp120 on transmission.
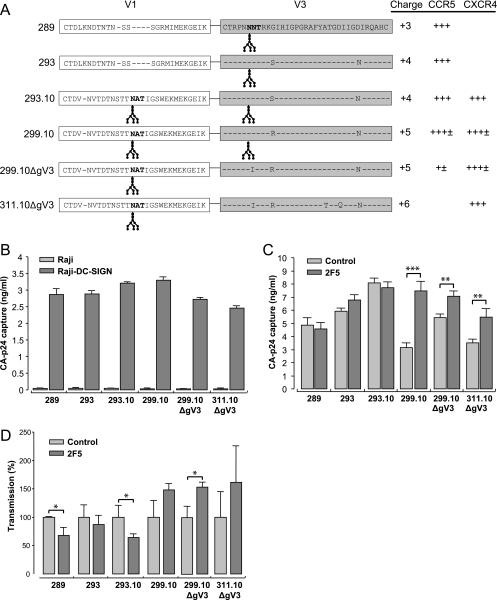
We analyzed transmission of R5 and X4 HIV-1 further by studying a panel of molecularly cloned viruses variant in their coreceptor phenotype. The gp120 envelope sequence of these virus clones was based on mutations found in envelope sequences in the V1 and V3 regions of gp120 from a patient progressing in AIDS. The viruses were composed of the LAI backbone with selected modifications in the V1 and V3 regions, as previously described (32). A V1 insertion provided an additional N-linked glycosylation site, and the V3 region increased in overall positive amino acid charge, with loss of an N-linked glycosylation site. The coreceptor usage phenotype of these viruses has been previously tested and is shown in Fig. 5A. These viruses represent a switch in coreceptor phenotype from that of R5 through R5X4 to X4.
FIG. 5.
Capture and transmission of R5X4 and X4 HIV-1 viruses by Raji-DC-SIGN cells are dependent on the amino acid sequences of the V1 and V3 loops on gp120. (A) Envelope sequence and coreceptor usage of the molecularly cloned HIV-1 variants (32). (B) Capture of viruses by Raji or Raji-DC-SIGN cells with a 15 ng/ml viral CA-p24 input. (C) Capture of control and 2F5-neutralized HIV-1 clones by Raji-DC-SIGN cells with a 40 ng/ml CA-p24 input. (D) Transmission of control and 2F5-neutralized HIV-1 clones by Raji-DC-SIGN cells. Transmission of neutralized HIV-1 was normalized to control HIV-1 and plotted as mean values of triplicates ± standard deviations. Data represent the results of three independent experiments. *, P ≤ 0.05; **, P ≤ 0.005; ***, P ≤ 0.0005. ± or + represents 0.5 log or log CA-p24 (ng/ml) HIV-1 production on U87.CD4 cells expressing either CCR5 or CXCR4.
Capture of the different molecularly cloned viruses by Raji-DC-SIGN or control cells was determined by measuring intracellular captured CA-p24 (Fig. 5B). Raji-DC-SIGN cells captured 2.5 to 3.3 ng/ml CA-p24 of all strains, and no virus uptake was seen with Raji cells. The two viruses with the additional glycosylation sites are the best captured, as previously reported (30). The effect of 2F5 Ab neutralization on capture of the different virus strains by Raji-DC-SIGN cells is shown in Fig. 5C. The two R5 strains 289 and 293 neutralized with 2F5 were as efficiently captured as untreated virus. No difference in DC-SIGN capture was observed for the 293.10 R5X4 strain upon 2F5 treatment, whereas a twofold increase (P < 0.0001) was observed with the R5X4 299.10 strain. This suggests that capture by DC-SIGN of 2F5-neutralized HIV-1 is influenced by an increase in the charge of the V3 loop. The 299.10ΔgV3 R5X4 strain and the 311.10ΔgV3 X4 strain were also more efficiently captured when neutralized by 2F5, (1.3 and 1.5-fold, respectively; P < 0.005). These results illustrate that HIV-1 X4 variants are more efficiently captured by DC-SIGN upon neutralization with the 2F5 Ab.
The effect of 2F5 neutralization on transmission by Raji-DC-SIGN of the molecularly cloned viruses is shown in Fig. 5D. Transmission of the 289, 293, and 293.10 strains was reduced by 33% (P < 0.05), 15%, and 37% (P < 0.05), respectively, upon treatment with 2F5. Neutralization with 2F5 of the 299.10, 299.10ΔgV3 (P < 0.05), and 311.10ΔgV3 strains increased transmission. From these results we conclude that molecular clones with an X4 or R5X4 phenotype with a higher V3 charge are preferentially transmitted upon neutralization with 2F5. Moreover, there is a link between capture and transmission of 2F5-neutralized virus, as seen with the R5 and X4 HIV-1 shown in Fig. 1.
DC-SIGN-expressing cells preferentially enhance CD4+ T-lymphocyte infection of X4 over R5 HIV-1.
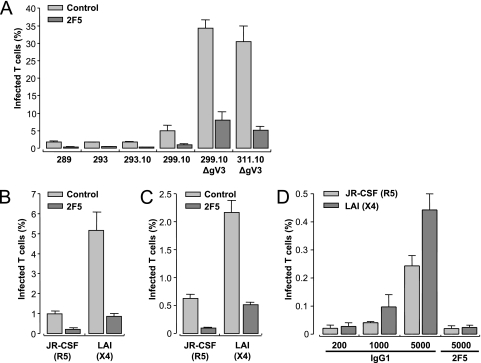
Reversion of neutralized HIV-1 to infectious HIV-1 upon transmission by DC-SIGN-expressing cells is more efficient for X4 viruses than R5 strains. The efficiency of transmission by Raji-DC-SIGN cells in the continuous presence of fully neutralizing concentrations of 2F5 was tested in an infection assay (Fig. 6). The 289, 293, and 293.10 viruses infected ∼1.7% of CD4+ T lymphocytes in the presence of Raji-DC-SIGN cells, whereas infection with the 299.10, 299.10ΔgV3, and 311.10ΔgV3 strains increased by 5%, 34%, and 30%, respectively (Fig. 6A). Upon neutralization with 2F5, CD4+ T lymphocytes showed higher levels of infection with the 299.10, 299.10ΔgV3, and 311.10dΔgV3 viruses (1%, 7.5%, and 5%, respectively) than the level of ∼0.35% infection with the 289, 293, and 299 strains. An overall neutralization of 80% was observed for all strains, presumably due to reneutralization of released particles. These results demonstrate that there is a preferential enhancement of transmission of X4 strains compared to R5-tropic HIV-1 by Raji-DC-SIGN cells in the absence or presence of NAb. We further analyzed the transmission by Raji-DC-SIGN cells (Fig. 6B) and iMDDCs (Fig. 6C) of JR-CSF and LAI with this infection assay. Infection of CD4+ T lymphocytes by LAI was more efficient than by JR-CSF in the presence of Raji-DC-SIGN cells (Fig. 6B) or iMDDCs (Fig. 6C) (five- and fourfold, respectively). Infection by neutralized X4 virus was also more efficient than with neutralized R5 virus for both types of DC-SIGN positive cells. The infection efficiency of JR-CSF and LAI on CD4+ T lymphocytes without DC-SIGN-expressing cells is illustrated in Fig. 6D. At low viral input (200 TCID50s) no significant infection could be detected for either strain. At high viral input (5,000 TCID50s) infection of CD4+ T lymphocytes was ∼0.25% for JR-CSF and about twofold higher for LAI (∼0.45%). These results illustrate that X4 HIV-1 infects CD4+ T lymphocytes more efficiently than R5 HIV-1, likely due to the level of coreceptor expression. Neutralization with 2F5, however, strongly blocked infection of CD4+ T lymphocytes at high viral input for both strains, which was not observed when Raji-DC-SIGN cells or iMDDCs were present in the coculture (Fig. 6B and C). These data illustrate that DC-SIGN-expressing cells are required to allow efficient HIV-1 infection of CD4+ T lymphocytes in the presence of NAbs.
FIG. 6.
DC-SIGN-expressing cells preferentially transmit viral variants, arising later in infection. (A) CD4+ T lymphocytes were cocultured with Raji-DC-SIGN cells and infected with molecularly cloned R5, early and late R5X4, or X4 HIV-1 in the absence or presence of 2F5 Ab. CD4+ T lymphocytes were cultured with Raji-DC-SIGN cells (B) or with iMDDCs (C) and infected with untreated or 2F5-neutralized R5 or X4 HIV-1. Data represent results of two independent experiments and were plotted as the mean of triplicates ± standard deviation as a percentage of infected CD4+ T lymphocytes. (D) Infection of CD4+ T lymphocytes by increasing amounts of R5 or X4 HIV-1 in the presence or absence of 2F5. Data represent means of quadruplicate experiments ± standard deviations at the indicated viral input.
Blocking DC-SIGN on iMDDCs strengthens HIV-1 neutralization by 2F5.
To investigate the role of DC-SIGN in HIV-1 infection of CD4+ T lymphocytes mixed with iMDDCs, we pretreated the iMDDCs with a DC-SIGN-blocking Ab and inoculated the cell mixture with HIV-1 variants in the presence or absence of 2F5 (Table 1). Blocking DC-SIGN on iMDDCs resulted in reduced infection by all viruses, with levels varying from 29 to 51%, which illustrates the importance of DC-SIGN in capture and transfer of HIV-1 to CD4+ T lymphocytes. The effect of 2F5 neutralization on CD4+ T lymphocyte infection with the different molecular strains varied between 60 to 86% and was on average 75%. The measured inhibition of infection when both 2F5 and anti-DC-SIGN were used for blocking proved to be higher, except for LAI, than that predicted from blocking with either antibody separately, indicating that DC-SIGN on iMDDCs reduces the efficacy of antibody neutralization.
TABLE 1.
The importance of DC-SIGN on iMDDCs in neutralization of R5, R5X4, or X4 HIV-1 by 2F5
| HIV-1 strain | Percentage of inhibition with the indicated treatmenta
|
|||
|---|---|---|---|---|
| 2F5 | DC-SIGN | 2F5 and DC-SIGN
|
||
| Expectedb | Measured | |||
| 289 | 72 ± 6.6 | 42 ± 9.7 | 84 | 90 ± 2.7 |
| 293 | 60 ± 5.1 | 29 ± 5.7 | 72 | 88 ± 8.5 |
| 293.10 | 77 ± 5.7 | 31 ± 12.0 | 84 | 93 ± 3.3 |
| 299.10 | 77 ± 14.5 | 31 ± 39.2 | 84 | 90 ± 19.6 |
| 299.10ΔgV3 | 75 ± 3.4 | 51 ± 24.3 | 88 | 90 ± 3.6 |
| 311.10ΔgV3 | 74 ± 22.4 | 41 ± 23.6 | 85 | 92 ± 8.1 |
| JR-CSF | 86 ± 3.2 | 37 ± 11.5 | 91 | 95 ± 1.2 |
| LAI | 72 ± 4.4 | 44 ± 4.8 | 84 | 80 ± 5.6 |
Virus was treated with IgG1 or 2F5 and/or DC-SIGN on iMDDCs was blocked; infection of CD4+ T lymphocytes was measured in presence of Ab. Data were analyzed and plotted as mean values of quadruplicates normalized to control HIV-1, ± standard deviation.
Expected inhibition = 2F5 percentage of neutralization + [(100% − 2F5 percentage of neutralization) × DC-SIGN percentage of inhibition].
DISCUSSION
Over the HIV-1 disease course the coreceptor phenotype of the virus switches from R5 to X4 in ∼50% of infected individuals. The utilization of the CXCR4 coreceptor by HIV-1 is associated with an accelerated CD4 cell decline and faster progression to AIDS, a critical step in pathogenesis (3). The factors contributing to the emergence of X4 variants are poorly understood but are believed to include target cell availability as well as immune selection through either innate or adaptive responses in the host (5, 38, 51).
In the present study we demonstrate that R5 viruses are relatively poorly transferred by DCs to CD4+ T lymphocytes in relation to X4 variants and that some gp41/120-binding Abs increase the transfer of X4 viruses. This enhancing effect is observed for cells expressing high levels of DC-SIGN such as iMDDCs and Raji-DC-SIGN cells. We also demonstrate that NAbs are more effective against blocking R5 than X4 viral transmission by iMDDCs and Raji-DC-SIGN cells. Our results with the DC-SIGN blocking Ab also indicate that this specific C-type lectin is important for mediating transfer of neutralized virus to CD4+ T lymphocytes. Overall, our results indicate that the interaction of HIV-1 with DCs, and especially those cells expressing DC-SIGN, may help explain the large increase in viral loads typically observed in patients undergoing a switch in viral coreceptor phenotype.
DC-SIGN interacts with glycan moieties present on the gp120 of HIV-1. We illustrate that all tested HIV-1 variants were efficiently captured by DC-SIGN; addition or removal of an N-linked glycosylation site in the V1 or V3 region of gp120 slightly modulated DC-SIGN capture as seen earlier (30), although this modulation was less pronounced due to a reduced viral input. Treatment of viruses with the 2F5 Ab increased capture by DC-SIGN on Raji cells (Fig. 1 and 5). This was, however, restricted to late R5X4 and X4 variants. iMDDCs demonstrated a trend in increased capture of LAI upon neutralization with 2F5 (data not shown). Although iMDDCs express high levels of DC-SIGN, the contribution of other cellular receptors could negate the enhanced capture of 2F5-neutralized HIV-1 by this specific C-type lectin. Most interesting, although mMDDCs express reduced levels of DC-SIGN compared to iMDDCs, they transmit R5 and X4 HIV-1 more efficiently (20, 41). mDCs express low levels of DC-SIGN but can also efficiently transmit virus in trans to susceptible T cells. These data, together with the result that blocking DC-SIGN on iMDDCs reduces infection of CD4+ T lymphocytes ∼40% (Table 1), illustrate that other cellular receptors on iMDDCs, mMDDCs, and mDCs can facilitate the capture and transmission of HIV-1. Furthermore, for both mMDDCs and mDCs no enhancement to transmission was observed when LAI was neutralized with 2F5, which reinforces the idea that DC-SIGN expression is correlated with enhanced transmission of such viruses.
As previously published, the Fc receptor facilitates the increase in capture of HIV-1 Ab immune complexes by DC-SIGN (50), but this capture cannot be mediated by the Fc receptor alone. Since neutralization with 2F5 should trigger the same enhanced capture by DC-SIGN for all viruses facilitated by the Fc receptor, we speculate that Abs increase the affinity of virus for DC-SIGN of the late R5X4 and X4 variants.
We previously reported that 2F5-neutralized HIV-1 can regain infectivity upon capture and transmission by DCs (50). Here, we show that neutralization with 2F5 increased transmission of only X4 and late R5X4 HIV-1 variants by DC-SIGN-expressing cells, whereas R5 virus transmission was decreased upon pretreatment with 2F5, indicating that a proportion of the R5 viruses remained neutralized during transmission. Similar to 2F5 and 4E10, the V3 Abs binding either the tip (V3-13 recognizing IRIQRGPGR sequence) or stem of the V3 loop (V3-23 recognizing INCTRPN sequence) increased transmission of X4 viruses and reduced R5 transfer. Although gp41-directed Abs are seldom or rarely found in serum from HIV-1 infected patients (21), neutralizing V3-directed Abs are present (28, 54), which could have an advantageous effect on selection of HIV-1 variants using CXCR4. Of all the gp120-directed Abs tested, only the b12 Ab prevented transmission of both R5 as well as X4 viruses, suggesting that the b12 Ab cannot be dissociated from HIV-1 following the interaction with DCs. The 2G12 Ab inhibited transmission of R5 as well as X4 viruses although to variant levels. This decreased transmission is more likely due to a reduced viral capture by DC-SIGN rather than an inhibition of infectious virus transmission, since 2G12 binding to gp120 is known to interfere with viral capture by DC-SIGN (4). In summary, the antibodies used have different capacities to interfere with or enhance viral capture and transfer by DCs; a complex interaction between the viral envelope and the Abs induced by the host will therefore determine the extent to which this mechanism contributes to viral replication and switching in coreceptor phenotype.
The mechanism underlying DC-mediated trans infection of CD4+ T lymphocytes is not known. It can be either through direct transfer of virus or via internalization of viral particles into intracellular compartments and their reemergence through the immunological synapse at the cell surface (24). If viral transmission occurs via internalization, X4 variants could be differently processed intracellularly than R5 variants, resulting in lower viral degradation and stronger antibody dissociation, with only the b12 Ab being attached during trafficking, in contrast to other Abs. However, it was recently suggested that the majority of trans infection of CD4+ T lymphocytes occurs through direct viral transfer without internalization (8, 23). In this scenario the interaction of the gp41/120 envelope with DC-SIGN or other cellular HIV-1 receptors on the plasma membrane should induce dissociation of Abs from the viral particle. Either way, the generation of b12-like Abs could prove to be effective in not only inhibiting virus transmission but also limiting viral propagation during disease progression. Means of induction of b12-like Abs should therefore be considered for both prophylactic as well as therapeutic vaccine strategies against HIV-1.
After DCs capture HIV-1 by interacting with surface C-type lectins (13) and specifically DC-SIGN on iDCs, the virus is targeted for degradation in lysosomes and processed into peptide for presentation by MHC molecules. The likelihood that iDCs loaded with HIV-1 in the epithelium encounter CD4+ T lymphocytes is limited, whereas it could be envisaged that in the lymph nodes DCs are continuously stimulated by CD4+ T lymphocytes, making transmission in trans more likely and thus providing a niche for X4 variants to emerge in the presence of Abs. We have previously hypothesized that early R5X4-switching viruses have an envelope structure with an open configuration that renders them more easily neutralized by CC chemokines or Abs (29), thereby providing a bottleneck to their emergence. This hypothesis has recently been supported by a study analyzing neutralization of biological clones generated from patients progressing in their disease course and undergoing a switch in coreceptor usage (6). Our results suggest a complex interaction between HIV-1 and DCs that could explain the virus's escape from strong NAb responses and the higher viral loads of the X4 phenotype.
We demonstrate that, compared to transmission of R5 viruses, DCs preferentially transmit X4 viruses to CD4+ T lymphocytes, which could have implications for disease progression. This mode of transmission is likely to play a role later in disease progression since the first HIV-1-specific Abs have to be made to block infection of DCs, thereby reducing cis transmission, which is dominated by R5 strains (15, 16). Furthermore, our results indicate that Abs could select for the earlier emergence of the more pathogenic X4 strains, providing a cautious note for the use of vaccines aimed at inducing HIV-1-specific humoral immune responses.
Acknowledgments
We thank B. Berkhout for helpful comments and critical reading of the manuscript, S. Heynen for performing the CA-p24 ELISA, and the NIH AIDS Research and Reference Reagent Program for supplying us with reagents and Abs (H. Katinger for 2F5, 4E10, and 2G12; J. Robinson for 1.7b and 4.8d; D. P. Burton and P. Parren for b12; and J. Laman for V3-13 and V3-21). We thank Theo Geijtenbeek for supplying us with the DC-SIGN Ab AZN-D1 and Tony van Capel for experimental advice and delivery of DC marker Abs.
Footnotes
Published ahead of print on 4 June 2008.
REFERENCES
- 1.Arrighi, J. F., M. Pion, E. Garcia, J. M. Escola, Y. van Kooyk, T. B. Geijtenbeek, and V. Piguet. 2004. DC-SIGN-mediated infectious synapse formation enhances X4 HIV-1 transmission from dendritic cells to T cells. J. Exp. Med. 2001279-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6200-206. [DOI] [PubMed] [Google Scholar]
- 3.Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17657-700. [DOI] [PubMed] [Google Scholar]
- 4.Binley, J. M., S. Ngo-Abdalla, P. Moore, M. Bobardt, U. Chatterji, P. Gallay, D. R. Burton, I. A. Wilson, J. H. Elder, and A. de Parseval. 2006. Inhibition of HIV Env binding to cellular receptors by monoclonal antibody 2G12 as probed by Fc-tagged gp120. Retrovirology 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaak, H., A. B. van't Wout, M. Brouwer, B. Hooibrink, E. Hovenkamp, and H. Schuitemaker. 2000. In vivo HIV-1 infection of CD45RA(+) CD4(+) T cells is established primarily by syncytium-inducing variants and correlates with the rate of CD4(+) T cell decline. Proc. Natl. Acad. Sci. USA 971269-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunnik, E. M., E. D. Quakkelaar, A. C. van Nuenen, B. Boeser-Nunnink, and H. Schuitemaker. 2007. Increased neutralization sensitivity of recently emerged CXCR4-using human immunodeficiency virus type 1 strains compared to coexisting CCR5-using variants from the same patient. J. Virol. 81525-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canque, B., Y. Bakri, S. Camus, M. Yagello, A. Benjouad, and J. C. Gluckman. 1999. The susceptibility to X4 and R5 human immunodeficiency virus-1 strains of dendritic cells derived in vitro from CD34(+) hematopoietic progenitor cells is primarily determined by their maturation stage. Blood 933866-3875. [PubMed] [Google Scholar]
- 8.Cavrois, M., J. Neidleman, J. F. Kreisberg, and W. C. Greene. 2007. In vitro derived dendritic cells trans-infect CD4 T cells primarily with surface-bound HIV-1 virions. PLoS Pathog. 3e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Witte, L., A. Nabatov, M. Pion, D. Fluitsma, M. A. de Jong, T. de Gruijl, V. Piguet, Y. van Kooyk, and T. B. Geijtenbeek. 2007. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat. Med. 13367-371. [DOI] [PubMed] [Google Scholar]
- 10.Ganesh, L., K. Leung, K. Lore, R. Levin, A. Panet, O. Schwartz, R. A. Koup, and G. J. Nabel. 2004. Infection of specific dendritic cells by CCR5-tropic human immunodeficiency virus type 1 promotes cell-mediated transmission of virus resistant to broadly neutralizing antibodies. J. Virol. 7811980-11987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia, E., M. Pion, A. Pelchen-Matthews, L. Collinson, J. F. Arrighi, G. Blot, F. Leuba, J. M. Escola, N. Demaurex, M. Marsh, and V. Piguet. 2005. HIV-1 trafficking to the dendritic cell-T-cell infectious synapse uses a pathway of tetraspanin sorting to the immunological synapse. Traffic 6488-501. [DOI] [PubMed] [Google Scholar]
- 12.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. Van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100587-597. [DOI] [PubMed] [Google Scholar]
- 13.Harman, A. N., J. Wilkinson, C. R. Bye, L. Bosnjak, J. L. Stern, M. Nicholle, J. Lai, and A. L. Cunningham. 2006. HIV induces maturation of monocyte-derived dendritic cells and Langerhans cells. J. Immunol. 1777103-7113. [DOI] [PubMed] [Google Scholar]
- 14.Hessell, A. J., L. Hangartner, M. Hunter, C. E. Havenith, F. J. Beurskens, J. M. Bakker, C. M. Lanigan, G. Landucci, D. N. Forthal, P. W. Parren, P. A. Marx, and D. R. Burton. 2007. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449101-104. [DOI] [PubMed] [Google Scholar]
- 15.Holl, V., M. Peressin, T. Decoville, S. Schmidt, S. Zolla-Pazner, A. M. Aubertin, and C. Moog. 2006. Nonneutralizing antibodies are able to inhibit human immunodeficiency virus type 1 replication in macrophages and immature dendritic cells. J. Virol. 806177-6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holl, V., M. Peressin, S. Schmidt, T. Decoville, S. Zolla-Pazner, A. M. Aubertin, and C. Moog. 2006. Efficient inhibition of HIV-1 replication in human immature monocyte-derived dendritic cells by purified anti-HIV-1 IgG without induction of maturation. Blood 1074466-4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu, J., M. B. Gardner, and C. J. Miller. 2000. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 746087-6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu, J., M. Pope, C. Brown, U. O'Doherty, and C. J. Miller. 1998. Immunophenotypic characterization of simian immunodeficiency virus-infected dendritic cells in cervix, vagina, and draining lymph nodes of rhesus monkeys. Lab. Investig. 78435-451. [PubMed] [Google Scholar]
- 19.Huber, M., M. Fischer, B. Misselwitz, A. Manrique, H. Kuster, B. Niederost, R. Weber, V. von Wyl, H. F. Gunthard, and A. Trkola. 2006. Complement lysis activity in autologous plasma is associated with lower viral loads during the acute phase of HIV-1 infection. PLoS Med. 3e441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izquierdo-Useros, N., J. Blanco, I. Erkizia, M. T. Fernandez-Figueras, F. E. Borras, M. Naranjo-Gomez, M. Bofill, L. Ruiz, B. Clotet, and J. Martinez-Picado. 2007. Maturation of blood-derived dendritic cells enhances human immunodeficiency virus type 1 capture and transmission. J. Virol. 817559-7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, Y., S. A. Migueles, B. Welcher, K. Svehla, A. Phogat, M. K. Louder, X. Wu, G. M. Shaw, M. Connors, R. T. Wyatt, and J. R. Mascola. 2007. Broad HIV-1 neutralization mediated by CD4-binding site antibodies. Nat. Med. 131032-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manrique, A., P. Rusert, B. Joos, M. Fischer, H. Kuster, C. Leemann, B. Niederost, R. Weber, G. Stiegler, H. Katinger, H. F. Gunthard, and A. Trkola. 2007. In vivo and in vitro escape from neutralizing antibodies 2G12, 2F5, and 4E10. J. Virol. 818793-8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marzi, A., D. A. Mitchell, C. Chaipan, T. Fisch, R. W. Doms, M. Carrington, R. C. Desrosiers, and S. Pohlmann. 2007. Modulation of HIV and SIV neutralization sensitivity by DC-SIGN and mannose-binding lectin. Virology 368322-330. [DOI] [PubMed] [Google Scholar]
- 24.McDonald, D., L. Wu, S. M. Bohks, V. N. KewalRamani, D. Unutmaz, and T. J. Hope. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 3001295-1297. [DOI] [PubMed] [Google Scholar]
- 25.Miller, C. J. 1998. Host and viral factors influencing heterosexual HIV transmission. Rev. Reprod. 342-51. [DOI] [PubMed] [Google Scholar]
- 26.Miller, C. J., and J. Hu. 1999. T cell-tropic simian immunodeficiency virus (SIV) and simian-human immunodeficiency viruses are readily transmitted by vaginal inoculation of rhesus macaques, and Langerhans' cells of the female genital tract are infected with SIV. J. Infect. Dis. 179(Suppl. 3)S413-S417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muster, T., F. Steindl, M. Purtscher, A. Trkola, A. Klima, G. Himmler, F. Ruker, and H. Katinger. 1993. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J. Virol. 676642-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nabatov, A. A., A. E. Masharsky, S. V. Verevochkin, A. V. Emelyanov, and A. P. Kozlov. 2004. Host-dependent serum specificity to the V3 domain of HIV-1. Scand. J. Immunol. 60471-476. [DOI] [PubMed] [Google Scholar]
- 29.Nabatov, A. A., G. Pollakis, T. Linnemann, A. Kliphius, M. I. Chalaby, and W. A. Paxton. 2004. Intrapatient alterations in the human immunodeficiency virus type 1 gp120 V1V2 and V3 regions differentially modulate coreceptor usage, virus inhibition by CC/CXC chemokines, soluble CD4, and the b12 and 2G12 monoclonal antibodies. J. Virol. 78524-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nabatov, A. A., T. van Montfort, T. B. Geijtenbeek, G. Pollakis, and W. A. Paxton. 2006. Interaction of HIV-1 with dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin-expressing cells is influenced by gp120 envelope modifications associated with disease progression. FEBS J. 2734944-4958. [DOI] [PubMed] [Google Scholar]
- 31.Ostrowski, M. A., S. J. Justement, A. Catanzaro, C. A. Hallahan, L. A. Ehler, S. B. Mizell, P. N. Kumar, J. A. Mican, T. W. Chun, and A. S. Fauci. 1998. Expression of chemokine receptors CXCR4 and CCR5 in HIV-1-infected and uninfected individuals. J. Immunol. 1613195-3201. [PubMed] [Google Scholar]
- 32.Pollakis, G., S. Kang, A. Kliphuis, M. I. Chalaby, J. Goudsmit, and W. A. Paxton. 2001. N-linked glycosylation of the HIV type-1 gp120 envelope glycoprotein as a major determinant of CCR5 and CXCR4 coreceptor utilization. J. Biol. Chem. 27613433-13441. [DOI] [PubMed] [Google Scholar]
- 33.Pope, M., M. G. Betjes, N. Romani, H. Hirmand, P. U. Cameron, L. Hoffman, S. Gezelter, G. Schuler, and R. M. Steinman. 1994. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell 78389-398. [DOI] [PubMed] [Google Scholar]
- 34.Purtscher, M., A. Trkola, A. Grassauer, P. M. Schulz, A. Klima, S. Dopper, G. Gruber, A. Buchacher, T. Muster, and H. Katinger. 1996. Restricted antigenic variability of the epitope recognized by the neutralizing gp41 antibody 2F5. AIDS 10587-593. [DOI] [PubMed] [Google Scholar]
- 35.Purtscher, M., A. Trkola, G. Gruber, A. Buchacher, R. Predl, F. Steindl, C. Tauer, R. Berger, N. Barrett, and A. Jungbauer. 1994. A broadly neutralizing human monoclonal antibody against gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 101651-1658. [DOI] [PubMed] [Google Scholar]
- 36.Randolph, G. J., V. Angeli, and M. A. Swartz. 2005. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 5617-628. [DOI] [PubMed] [Google Scholar]
- 37.Reece, J. C., A. J. Handley, E. J. Anstee, W. A. Morrison, S. M. Crowe, and P. U. Cameron. 1998. HIV-1 selection by epidermal dendritic cells during transmission across human skin. J. Exp. Med. 1871623-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Regoes, R. R., and S. Bonhoeffer. 2005. The HIV coreceptor switch: a population dynamical perspective. Trends Microbiol. 13269-277. [DOI] [PubMed] [Google Scholar]
- 39.Rubbert, A., C. Combadiere, M. Ostrowski, J. Arthos, M. Dybul, E. Machado, M. A. Cohn, J. A. Hoxie, P. M. Murphy, A. S. Fauci, and D. Weissman. 1998. Dendritic cells express multiple chemokine receptors used as coreceptors for HIV entry. J. Immunol. 1603933-3941. [PubMed] [Google Scholar]
- 40.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 1791109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanders, R. W., E. C. de Jong, C. E. Baldwin, J. H. Schuitemaker, M. L. Kapsenberg, and B. Berkhout. 2002. Differential transmission of human immunodeficiency virus type 1 by distinct subsets of effector dendritic cells. J. Virol. 767812-7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanders, R. W., M. Venturi, L. Schiffner, R. Kalyanaraman, H. Katinger, K. O. Lloyd, P. D. Kwong, and J. P. Moore. 2002. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J. Virol. 767293-7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shibata, R., T. Igarashi, N. Haigwood, A. Buckler-White, R. Ogert, W. Ross, R. Willey, M. W. Cho, and M. A. Martin. 1999. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat. Med. 5204-210. [DOI] [PubMed] [Google Scholar]
- 44.Smed-Sorensen, A., K. Lore, J. Vasudevan, M. K. Louder, J. Andersson, J. R. Mascola, A. L. Spetz, and R. A. Koup. 2005. Differential susceptibility to human immunodeficiency virus type 1 infection of myeloid and plasmacytoid dendritic cells. J. Virol. 798861-8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sonza, S., A. Maerz, S. Uren, A. Violo, S. Hunter, W. Boyle, and S. Crowe. 1995. Susceptibility of human monocytes to HIV type 1 infection in vitro is not dependent on their level of CD4 expression. AIDS Res. Hum. Retrovir. 11769-776. [DOI] [PubMed] [Google Scholar]
- 46.Stiegler, G., R. Kunert, M. Purtscher, S. Wolbank, R. Voglauer, F. Steindl, and H. Katinger. 2001. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 171757-1765. [DOI] [PubMed] [Google Scholar]
- 47.Sugaya, M., K. Lore, R. A. Koup, D. C. Douek, and A. Blauvelt. 2004. HIV-infected Langerhans cells preferentially transmit virus to proliferating autologous CD4+ memory T cells located within Langerhans cell-T cell clusters. J. Immunol. 1722219-2224. [DOI] [PubMed] [Google Scholar]
- 48.Trkola, A., H. Kuster, P. Rusert, B. Joos, M. Fischer, C. Leemann, A. Manrique, M. Huber, M. Rehr, A. Oxenius, R. Weber, G. Stiegler, B. Vcelar, H. Katinger, L. Aceto, and H. F. Gunthard. 2005. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat. Med. 11615-622. [DOI] [PubMed] [Google Scholar]
- 49.Turville, S. G., J. J. Santos, I. Frank, P. U. Cameron, J. Wilkinson, M. Miranda-Saksena, J. Dable, H. Stossel, N. Romani, M. Piatak, Jr., J. D. Lifson, M. Pope, and A. L. Cunningham. 2004. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood 1032170-2179. [DOI] [PubMed] [Google Scholar]
- 50.van Montfort, T., A. A. Nabatov, T. B. Geijtenbeek, G. Pollakis, and W. A. Paxton. 2007. Efficient capture of antibody neutralized HIV-1 by cells expressing DC-SIGN and transfer to CD4+ T lymphocytes. J. Immunol. 1783177-3185. [DOI] [PubMed] [Google Scholar]
- 51.van Rij, R. P., H. Blaak, J. A. Visser, M. Brouwer, R. Rientsma, S. Broersen, A. M. Roda Husman, and H. Schuitemaker. 2000. Differential coreceptor expression allows for independent evolution of non-syncytium-inducing and syncytium-inducing HIV-1. J. Clin. Investig. 1061039-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422307-312. [DOI] [PubMed] [Google Scholar]
- 53.Zaitseva, M., A. Blauvelt, S. Lee, C. K. Lapham, V. Klaus-Kovtun, H. Mostowski, J. Manischewitz, and H. Golding. 1997. Expression and function of CCR5 and CXCR4 on human Langerhans cells and macrophages: implications for HIV primary infection. Nat. Med. 31369-1375. [DOI] [PubMed] [Google Scholar]
- 54.Zwart, G., T. F. Wolfs, M. Valk, H. L. Van der, C. L. Kuiken, and J. Goudsmit. 1992. Characterization of the specificity of the human antibody response to the V3 neutralization domain of HIV-1. AIDS Res. Hum. Retrovir. 81897-1908. [DOI] [PubMed] [Google Scholar]
- 55.Zwick, M. B., A. F. Labrijn, M. Wang, C. Spenlehauer, E. O. Saphire, J. M. Binley, J. P. Moore, G. Stiegler, H. Katinger, D. R. Burton, and P. W. Parren. 2001. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 7510892-10905. [DOI] [PMC free article] [PubMed] [Google Scholar]