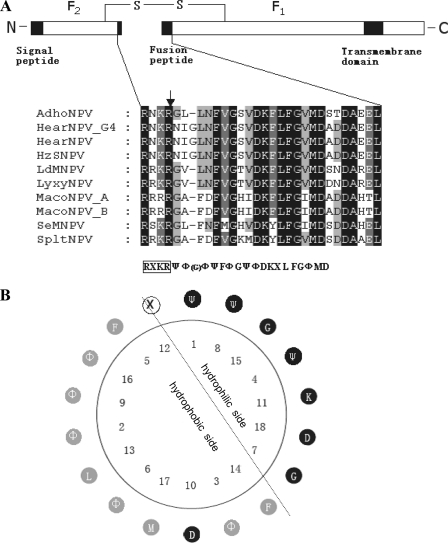
FIG. 1.
Presentation of group II F protein and fusion peptides. (A) Schematic presentation of group II F protein and multiple sequence alignment of the putative fusion peptides. The line represents the disulfide-linked F protein subunits F1 and F2. The signal peptide, fusion peptide, and transmembrane domain are represented by black boxes. The expanded section shows a comparison of the amino acid sequences of the fusion peptides of group II F proteins. Alignment columns are shaded according to conservation (black, 100% identity; gray, 60 to 80% identity). Below the alignment is the consensus sequence. Ψ represents hydrophilic amino acids, Φ represents hydrophobic amino acids, X represents any amino acid, and G in the parentheses represents the extra amino acid in the fusion peptides of HearNPV and HzNPV. The consensus furin recognition sequence is boxed, and the arrow indicates the cleavage site. (B) Helical wheel presentation of the putative fusion peptide of group II NPV F proteins. Hydrophilic residues are shown in black, hydrophobic residues are shown in gray, and any residues in white. The simplest amino acid G with an “-H” side chain is classified as hydrophilic. The dotted line represents the division between the hydrophilic face and the hydrophobic face of the amphiphilic residues. The fusion peptide is numbered from the N terminus of the cleaved F1 fragment.