FIG. 1.
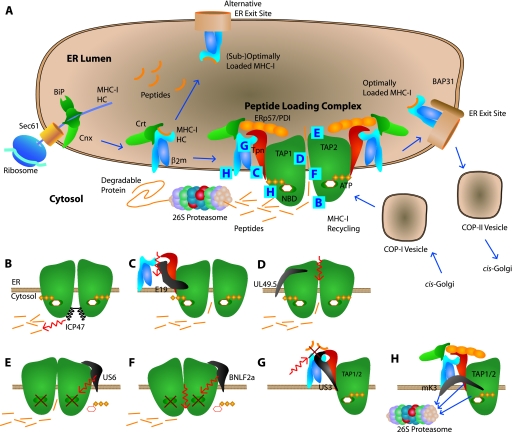
MHC-I maturation and virus proteins interfering with the peptide-loading complex. (A) Maturation of MHC-I in the ER starts in a specific way for all MHC-I molecules. The nascent MHC-I heavy chain is translated into the ER lumen through the Sec61 translocon. BiP and calnexin (Cnx) assist in the initial folding of the MHC-I heavy chain, allowing it to bind β2-microglobulin (β2m). After β2-microglobulin binding, the MHC-I molecule binds to calreticulin (Crt). At this intermediate processing stage, the MHC-I molecule may have already acquired a peptide able to induce final maturation; alternatively, the MHC-I may be from a HLA allele less prone to binding the peptide-loading complex or may be unable to bind the peptide-loading complex due to VIPR action, resulting in ER exit in either case. Other MHC-I molecules bind to tapasin (Tpn) and are allowed to mature in the peptide-loading complex, which consists of at least TAP, tapasin, calreticulin, ERp57, and protein disulfide isomerase (PDI). In the peptide-loading complex, tapasin mediates quality control, which ensures the loading of optimal peptides on MHC-I. A proportion of immature tapasin-associated MHC-I molecules escape ER retention but are transported back to the ER from the Golgi compartment in COP-I vesicles. Exit of optimally loaded MHC-I from the ER takes place at specialized ER exit sites. The MHC-I molecules are transported along the secretory pathway in COP-II vesicles and finally egress to the cell surface. (B) Herpes simplex virus ICP47 prevents peptides from binding to TAP. (C) Adenovirus E19 blocks the MHC-I-tapasin interaction and thereby prevents its integration into the peptide-loading complex. (D) Bovine herpes virus UL49.5 allows ATP and peptide binding to TAP but still inhibits the translocation of peptides into the ER. (E) Human cytomegalovirus US6 is an ER luminal protein that prevents ATP binding to TAP on the cytosolic nucleotide binding domain (NBD). (F) Epstein-Barr virus BNFL2a prevents both ATP and peptide binding to TAP. (G) Human cytomegalovirus US3 interacts directly with tapasin and interferes with tapasin-mediated quality control in the peptide-loading complex. (H) Herpes simplex virus type 68 mK3 integrates into a structurally intact peptide-loading complex, thereby mediating the ubiquitination of MHC-I, tapasin, and TAP, leading to proteasomal degradation.