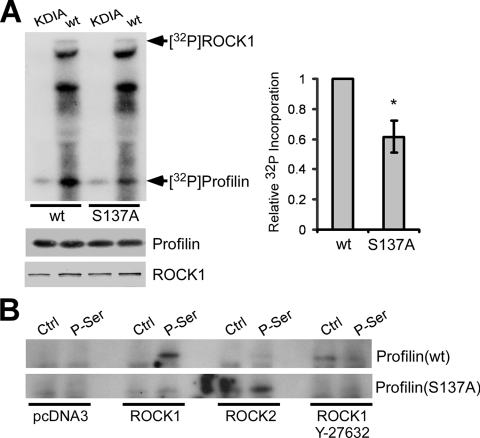
FIG. 4.
ROCK1 phosphorylates profilin at Ser-137 in vitro and in vivo. (A) Immunoprecipitated Myc-tagged ROCK1(wt) or ROCK1(KDIA) was mixed with profilin(wt) or profilin(S137A) in an in vitro kinase assay and analyzed by autoradiography and Western blotting. While the kinase-dead ROCK1(KDIA) caused little 32P incorporation into profilin, ROCK1(wt) phosphorylated profilin(wt) and, to a 40% lesser extent, profilin(S137A) (average of three experiments) (*, P < 0.05 by unpaired t test). Error bars represent the SEM. (B) Myc-tagged profilin(wt) or profilin(S137A) was cotransfected into HEK293 cells with ROCK1, ROCK2, or pcDNA3 and treated with or without 50 μM Y-27632 for 24 h. Immunoprecipitation was performed with a phosphoserine-specific antibody (P-Ser) or normal IgG (control [Ctrl]), followed by Western blotting for the Myc tag. No profilin(wt) bound to the P-Ser antibody without ROCK1, whereas ROCK1 overexpression increased binding, which was inhibited by Y-27632. Profilin(S137A) failed to bind the P-Ser antibody regardless of ROCK1 overexpression. ROCK2 overexpression did not increase the binding of profilin(wt) to the P-Ser antibody but increased the binding of profilin(S137A).