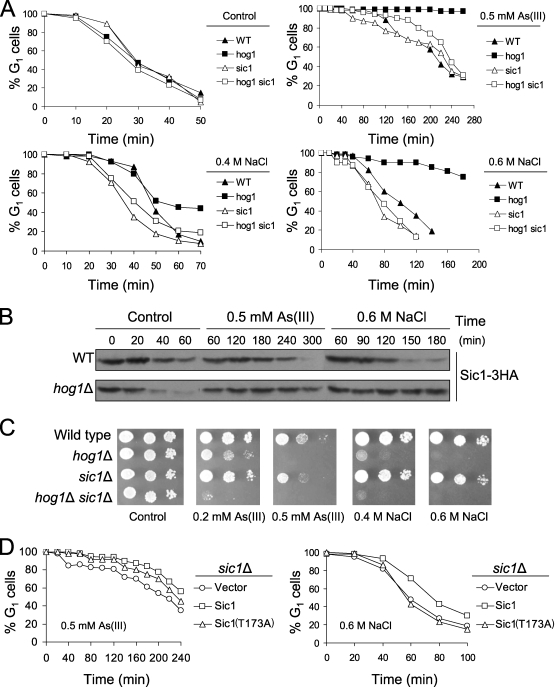
FIG. 4.
Hog1 triggers Sic1 degradation to promote adaptation to stress-induced G1 checkpoint arrest. (A) Deletion of SIC1 suppresses prolonged G1 delay of hog1Δ mutant in the presence of arsenite and sodium chloride. G1-synchronized cells were released in the presence or absence of 0.5 mM arsenite or 0.4 M or 0.6 M NaCl and analyzed by using an α-factor-nocodazole trap assay. (B) Sic1 remains stable in hog1Δ cells during arsenite and hyperosmotic stress. Cells were treated as described above, and Sic1 levels were monitored by Western blot analysis using an anti-HA antibody. (C) Bypass of persistent G1 arrest by deletion of SIC1 does not increase tolerance of hog1Δ cells for arsenite and sodium chloride. (D) Expression of Sic1(T173A) mutant lacking the Hog1 phosphorylation site does not phenocopy the cell cycle defect of hog1Δ mutation. Cells with plasmid pCM189 (vector [control]), pCM189-SIC1-4HA, or pCM189-SIC1T173A-4HA were synchronized in G1 with α-factor, exposed to 2 μg/ml doxycycline for 30 min to turn off expression of SIC1, washed, released in fresh medium containing 2 μg/ml doxycycline in the presence or absence of 0.5 mM arsenite or 0.6 M NaCl, and assayed by α-factor-nocodazole trapping. As(III), arsenite; WT, wild type.