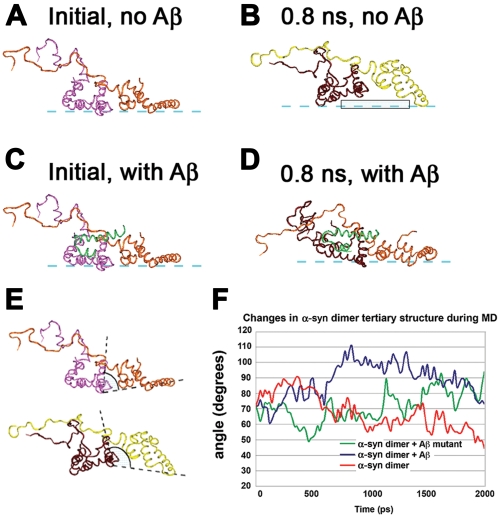
Figure 4. Molecular dynamics of conformational changes of membrane-associated α-syn in the presence of one Aβ monomer.
(A) Initial conformation of α-syn dimer on the membrane without Aβ. (B) 0.8 ns conformation of α-syn dimer on the membrane without Aβ. (C) Initial conformation of α-syn dimer on the membrane with Aβ monomer. (D) When complexed with the Aβ monomer, after 0.8 ns the conformation of the α-syn dimer on the membrane is drawn closer to the membrane, and the α-syn molecules make more contact points with the membrane surface than the α-syn dimer alone. (E) Comparison of the two complexes at 0.8 ns demonstrating the angle measured between the C-alpha atoms of the residues VAL66 (α-syn1), LYS45 (α-syn1), VAL37 (α-syn2). The complex without Aβ had an angle of <80° (upper), while the complex with Aβ had an angle of >80° (lower) and was more stable on the membrane. (F) Changes in angle measurements in the complexes over time without Aβ or in the presence of wild-type or mutated full-length Aβ. In the mutated Aβ peptide, positively-charged residues were substituted for negative ones and hydrophobic residues for hydrophilic ones (PHE4SER, GLU11ARG, VAL12SER, LYS16ASP).