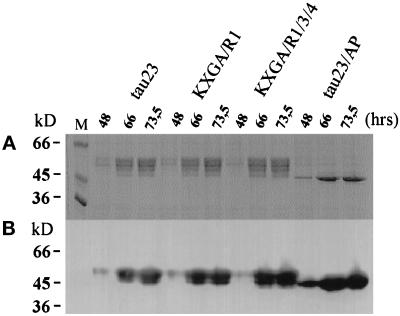
Figure 4.
Phosphorylation of tau and tau constructs transfected in Sf9 cells. (A) 10% SDS-PAGE. Tau protein or mutants (tau23, KXGA/R1, KXGA/R1/3/4, and tau23/AP) were isolated from equal quantities of Sf9 cells transfected with the appropriate tau constructs and prepared in equal volumes of sample buffer. Equal volumes of protein solution representing the different harvest time points were loaded onto the SDS gel (10%). The amounts of loaded proteins correspond approximately to 0.4 μg (48 h), 2.5 μg (66 h), and 3 μg (73.5 h). As seen from the multiple and strongly shifted bands, tau23 is highly phosphorylated in a heterogeneous manner. The same applies to contructs KXGA/R1 and KXGA/R1/3/4 (which lack only one or three phosphorylation sites at KXGS motifs but retain all SP or TP sites). By contrast, construct tau23/AP shows very little phosphorylation and no shift, because it lacks most phosphorylation sites (note that the KXGS sites do not induce a shift; Drewes et al., 1995). By implication, the strong shift sites S409 and S416 (targets of PKA or CaMKII) are also not phosphorylated in Sf9 cells. (B) The same samples were immunoblotted with antibody 5E2, which reacts independently of phosphorylation and reflects the total amount of tau protein. Lane M, marker proteins.