Abstract
Cytosolic phospholipase A2α (cPLA2α) activation is a regulatory step in the control of arachidonic acid (AA) liberation for eicosanoid formation. Sphingosine 1-phosphate (S1P) is a bioactive lipid mediator involved in the regulation of many important proinflammatory processes and has been found in the airways of asthmatic subjects. We investigated the mechanism of S1P-induced AA release and determined the involvement of cPLA2α in these events in A549 human lung epithelial cells. S1P induced AA release rapidly within 5 min in a dose- and time-dependent manner. S1P-induced AA release was inhibited by the cPLA2α inhibitors methyl arachidonyl fluorophosphonate (MAFP) and pyrrolidine derivative, by small interfering RNA-mediated downregulation of cPLA2α, and by inhibition of S1P-induced calcium flux, suggesting a significant role of cPLA2α in S1P-mediated AA release. Knockdown of the S1P3 receptor, the major S1P receptor expressed on A549 cells, inhibited S1P-induced calcium flux and AA release. The S1P-induced calcium flux and AA release was associated with sphingosine kinase 1 (Sphk1) expression and activity. Furthermore, Rho-associated kinase, downstream of S1P3, was crucial for S1P-induced cPLA2α activation. Our data suggest that S1P acting through S1P3, calcium flux, and Rho kinase activates cPLA2α and releases AA in lung epithelial cells. An understanding of S1P-induced cPLA2α activation mechanisms in epithelial cells may provide potential targets to control inflammatory processes in the lung.
Keywords: eicosanoid, calcium flux, airway inflammation
phospholipase a2 (PLA2) comprises a superfamily of enzymes that hydrolyze the ester bond of phospholipids at the sn-2 position (10, 38). It is well established that the hydrolytic action of arachidonic acid (AA)-containing phospholipids by cytosolic phospholipase A2α (cPLA2α) can constitute the first regulatory step of the eicosanoid biosynthetic cascade (5, 15). cPLA2α is unique among all the PLA2 family members (43) due to its substrate selectivity for AA-containing phospholipids, its regulation by submicromolar concentrations of Ca2+, and phosphorylation by mitogen-activated protein kinases (MAP kinases) (8, 11, 12, 22). Free AA generated by activated cPLA2α can be oxygenated into a variety of compounds including prostaglandins, thromboxanes, HETEs, and leukotrienes, which not only are involved in acute and chronic inflammatory diseases such as arthritis, asthma, and postischemic tissue injury, but can also regulate physiological processes, such as renal function, and female reproductive events including parturition (5, 6, 15, 49). The activity of cPLA2α is regulated by at least two major mechanisms that can act separately or in conjunction with each other. The first is by increasing intracellular Ca2+ concentration, which leads to the translocation of cPLA2α from cytosol to its preferential substrate phospholipids in the Golgi, ER, and nuclear membrane (41). The second regulatory mechanism of cPLA2α is through phosphorylation of one or more serine residues. It appears that phosphorylation leads to an activation of cPLA2α by increasing the specific activity of the enzyme (14). ERK1/2 and p38 MAP kinase-dependent phosphorylation are reported to regulate the phosphorylation and activation of cPLA2α in many cell lines (32, 42, 51, 55).
Sphingosine 1-phosphate (S1P) is a bioactive sphingolipid derived from sphingomyelin that is released by activated platelets and many other cells in response to a wide array of stimuli (16, 40). Biological functions of S1P signaling range from cell growth and survival to effector functions, such as proinflammatory mediator synthesis (9, 45, 48). S1P activates intracellular signaling pathways by binding to five G protein-coupled receptors designated S1P1-5, also known as endothelial differentiation gene EDG 1, 5, 3, 6, and 8 (17). Recently, S1P was identified as an intracellular “second messenger” (47, 53), and its intracellular generation is related to sphingosine kinase 1 (Sphk1) and lipid phosphate phosphatase-1 (LPP-1) in a human lung endothelial cell model (54). However, the relevant molecular targets with which it might interact within the cell remain to be defined.
The production of S1P is highly regulated by Sphk type 1 and 2, the two enzymes identified so far in mammals that produce S1P by ATP-dependent phosphorylation of sphingosine (25). In particular, Sphk1 expression is highest in the lung and spleen, whereas Sphk2 is most abundant in the liver and heart (24). Sphk1 activity is thought to be associated with some immunoregulatory events, such as mast cell degranulation and migration (18), activation of MAP kinase in HEK-293 and in T24 prostate carcinoma cells, and COX-2 activation and PGE2 formation in A549 epithelial cells (3). S1P is constitutively found in nanomolar-to-micromolar concentrations in human plasma and serum and has been shown to be an angiogenic factor and the primary endothelial cell chemotactic factor (20, 26). In addition to these vascular effects of S1P, reports in nonvascular tissue have described proinflammatory effects with IL-6 secretion in airway smooth muscle cells (2) and dendritic cells (31) and increased expression of IL-8 in human bronchial epithelial cells (9). Elevated S1P levels have been measured in the airways of asthmatic (but not control) subjects following segmental antigen challenge in association with inflammatory cell and protein influx (19). A study of S1P3-null and wild-type mice demonstrated that S1P acting on the S1P3 receptor expressed on lung epithelial cells but not vascular endothelium induces pulmonary edema by acute tight junction opening (13). Thus, it appears that extracellular S1P is a mediator of inflammation by regulating proinflammatory cytokine and chemokine production and epithelial tight junctions. S1P-induced AA release in A549 human lung adenocarcinoma cells has been reported (50). However, little is known about the mechanism of S1P-induced AA release, and its regulatory role on PLA2 activation remains to be defined.
We investigated the signaling pathways of S1P-induced PLA2 activation and AA release in the A549 human lung epithelial cell line. We show that cPLA2α is the major PLA2 that is activated by S1P to release AA. Our results demonstrate that the sphingolipid, S1P, releases AA mainly by activating cPLA2α through its receptor S1P3 to mobilize calcium and activate its downstream signaling. The small GTPase Rho, downstream of G12/13, is likely essential for cPLA2 activation. The S1P-induced calcium flux is related to newly formed S1P by Sphk1, which is essential for S1P-induced cPLA2α activation and AA release.
MATERIALS AND METHODS
Chemicals and reagents.
Cell culture medium and its supplements were obtained from Invitrogen/Gibco BRL (Carlsbad, CA). 5,6,8,9,11,12,14,15-3H-arachidonic acid (3H-arachidonic acid) was purchased from Amersham (Piscataway, NJ). MAFP, U73122, 2-aminoethoxydiphenylborate (2-APB), N,N-dimethylsphingosine (DMS), bromoenol lactone (BEL), N-{(2S,4R)-4-(Biphenyl-2-ylmethyl-isobutyl-amino)-1-[2-(2,4-difluorobenzoyl)-benzoyl]-pyrrolidin-2-ylmethyl}-3-[4-(2,4-dioxothiazolidin-5-ylidenemethyl)-phenyl]acrylamide, HCl (1,2,4-trisubstituted pyrrolidine derivative), SB-203580, SB-202109, PD-98059, 1,4-diamino-2,3-dicyano-1,4-bis-(2-aminophenylmercapto) butadiene (U0126), H1152, and pertussis toxin (Ptx) were obtained from Calbiochem (La Jolla, CA). Protease inhibitor cocktail is from Roche Applied Science (Indianapolis, IN); S1P, BSA, A23187, BAPTA-AM, EGTA, Y27632, Triton X-100, methyl acetate, DMSO, sodium vanadate, and okadaic acid were purchased from Sigma (St. Louis, MO).
Cell culture.
The A549 cell line was obtained from American Type Culture Collection (Rockville, MD), and cells were grown in Ham's F-12K media with 10% FBS and 2 mM of l-glutamine, in a humidified atmosphere of 95% air and 5% CO2.
AA release.
Whole cell AA release was performed as described by Pawliczak et al. (33). Briefly, the cells were grown in 12-well dishes (∼60% confluent) overnight and then were labeled with 0.5 μCi/ml of 5,6,8,9,11,12,14,15-3H-arachidonic acid (3H-AA) in 2% FBS culture media for 18 h. Cells were then washed three times with serum-free F-12K media. Subsequently, cells were stimulated with different concentrations of S1P in 1% BSA or vehicle (1% BSA alone) for different intervals as indicated. At the end of incubation, the medium was removed and centrifuged at 1,000 g for 5 min to remove any cells. An aliquot of media was transferred to scintillation vials containing 10 ml of Bio-Safe II scintillation fluid (Research International Products, Mount Prospect, IL) and counted in an LS6500 scintillation counter (Beckman, Fullerton, CA).
Western blot analysis.
Cells grown in six-well plates were placed in 2% FBS culture media for 18 h and then treated with stimuli as indicated. Cells were then washed with ice-cold PBS and harvested with lysis buffer (50 mM Tris·HCl, pH 7.5, 1 mM EDTA, 1% Triton X-100) containing 1 mM PMSF, protease inhibitor cocktails (Roche, Basel, Switzerland), 1 mM sodium vanadate, and 200 nM okadaic acid. The cell lysates were placed on ice for 30 min and then centrifuged at 13,000 rpm for 20 min. Protein concentration of cell lysates was determined by BCA kit (Pierce, Rockford, IL). Equal amounts of lysate proteins (20 μg) were resolved by 4–12% NuPAGE Bis-Tris gels or 10% Tris-glycine gel (Invitrogen, Carlsbad, CA) and then transblotted to nitrocellulose membranes (Invitrogen). The membranes were blocked with 5% nonfat skim milk in PBST (phosphate buffer saline containing 0.1% Tween 20) for 2 h and then incubated with primary antibodies: phospho-cPLA2, cPLA2, ERK1/2, phospho-ERK1/2 and p38, phospho-p38 (Cell Signaling Technology, Danvers, MA) overnight at 4°C. Subsequently, the membranes were washed and then incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Lab, West Grove, PA) for 1 h. The blots were developed using the ECL chemiluminescent kit (Amersham Biosciences) and visualized on a Kodak Image Station 440CF.
Transfection of small interfering RNAs for cPLA2α, iPLA2, S1P3, and Sphk1.
Small interfering RNA (siRNA) for cPLA2α was synthesized by IDT (Coralville, IA). The target site in cPLA2α is 5′ AAC UCU AGG GAC AGC AAC AUU 3′, which corresponds to bases 299–319 of cPLA2α coding sequence (GenBank acc. no. M68874). The single-stranded siRNAs were annealed by incubating a 100-μM concentration of each single strand in annealing buffer (100 mM potassium acetate, 30 mM HEPES, pH 7.4, 2 mM magnesium acetate) for 2 min at 90°C and slowly cooled down to room temperature. siRNAs for S1P3, iPLA2, Sphk1, and negative control siRNA were predesigned siRNAs purchased from Ambion (Austin, TX). Cells were seeded in 96-well plates for calcium flux measurement, in 12-well plates for AA release, or in 6-well plates for Western blot analysis or total RNA isolation. Cells were grown to 30–40% confluence and transfected using oligofectamine and Opti-MEM I reduced serum medium (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions using 20 nM final concentrations of siRNAs.
Real-time PCR.
Total RNA was extracted from A549 cells using QIA Shredder columns and RNeasy kits and was treated with DNase (Qiagen, Valencia, CA) and quantitated using a NanoDrop spectrophotometer (BioLabNet, Great Falls, VA). mRNA expression for selected genes was measured using real-time PCR performed on an ABI Prism 7900 sequence detection system (Applied Biosystems, Foster City, CA) using the following commercially available probe and primers sets (Applied Biosystems): S1P1-Hs00173499_m1, S1P2-Hs00244677_s1, S1P3-Hs00245464_s1, S1P4-Hs00269446_s1, S1P5-Hs00258220_s1, iPLA2-Hs00185926_m1, and Sphk1-Hs00184211_m1. cPLA2α was detected by a custom primer set: forward-5′TGGTGAGTGATTCAGCTTTATTCAA3′ and reverse-5′TGTGGCAAAGTCACTCAAAGGA and probe sequence: 6FAMCACAACTTCATGCTGGGCTTGAATCTCATAMRA (Applied Biosystems). Reverse transcription and real-time PCR were performed with an RT kit and TaqMan Universal PCR master mix (Applied Biosystems) according to the manufacturer's directions. Gene expression levels were presented as relative mRNA copy numbers compared with the internal control of GAPDH expression level and calculated as copy numbers compared with the least abundant transcript, which was designated as 1.
Calcium mobilization assay.
Calcium mobilization experiments were conducted using a FLIPR Calcium 3 assay kit (Molecular Devices, Sunnyvale, CA) according to the manufacturer's instructions. A549 cells (2 × 105 cells/well) were plated into 96-well plates and incubated in 10% FBS culture medium overnight and then replaced with 2% FBS culture medium with 20 mM HEPES for 18 h. Cells were incubated with Calcium 3 assay reagents for 1 h at 37°C, and fluorescence was measured every 4 s using the FlexStation (Molecular Devices).
Statistics.
Statistical analyses were done by Student's t-test or analysis of variances as indicated. A P value of <0.05 was considered statistically significant.
RESULTS
S1P induces the release of AA in A549 cells.
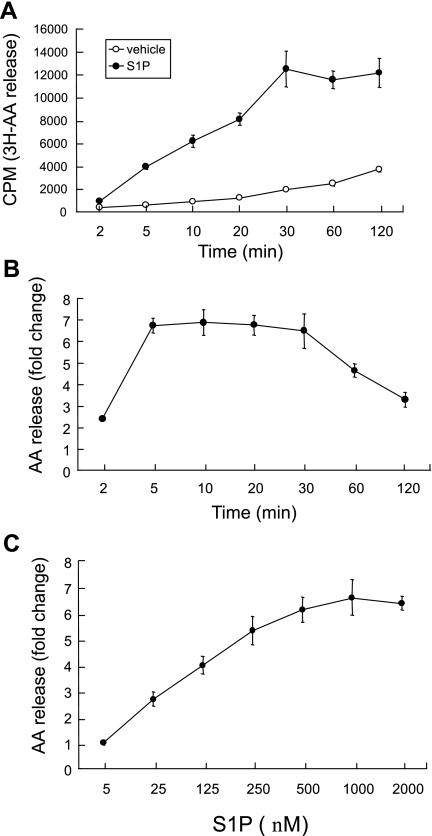
We studied the effect of S1P on AA release from A549 cells. The cells were labeled with 3H-AA and stimulated with S1P. A time course of AA mobilization was evaluated by measuring the accumulation of the labeled fatty acid in the medium. As shown in Fig. 1, A and B, S1P elicited a time-dependent increase in AA release, with the onset at 5 min and maximum at 10 min, followed by prolonged release up to 30 min. The S1P-induced response also exhibited concentration dependence (Fig. 1C). Within 10 min of incubation, S1P at 25 nM caused AA release with a maximum effect observed at 0.5 μM (Fig. 1C). The findings suggest that S1P is an agonist that might activate PLA2 or PLC pathways to release AA from phospholipids or diacylglycerol. Furthermore, its effect is an early phase event that might be regulated by signal transduction pathways through the S1P receptors.
Fig. 1.
Sphingosine 1-phosphate (S1P) induces arachidonic acid (AA) release in A549 cells. Cells labeled with 3H-AA were stimulated with 500 nM S1P or vehicle for 10 min, and 3H-AA released into the medium was measured at the indicated time (CPM, counts per minute) (A) or presented as fold change compared with the vehicle-treated cells (B). The cells were treated with the indicated concentrations of S1P for 10 min, and AA release was presented as the fold change compared with the vehicle-treated control cells (C). The data represent the means ± SD of 6 samples from a single experiment representative of 3 independent experiments that gave similar results. P < 0.01 across indicated time points (2–120 min) compared with vehicle-treated cells by Student's t-test. The dose response in C was statistically significant by 1-way ANOVA, P < 0.0001.
cPLA2α is the major phospholipase contributing to S1P-induced AA release.
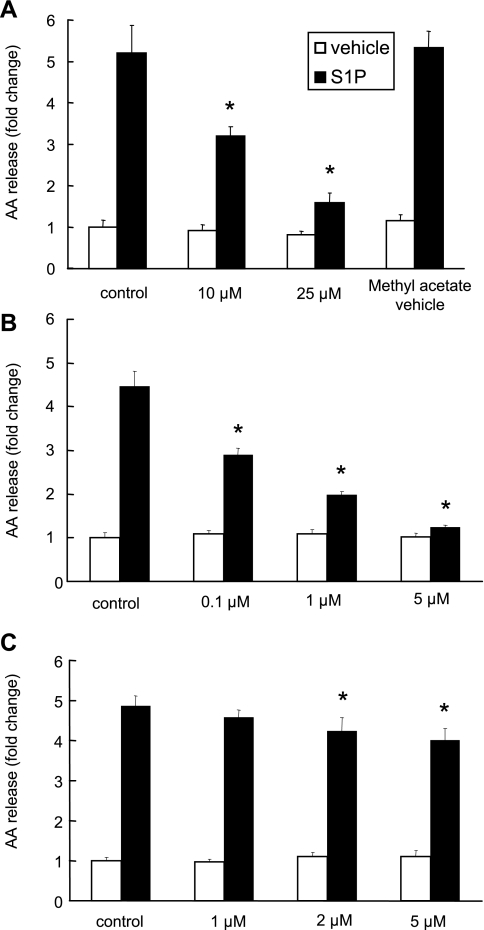
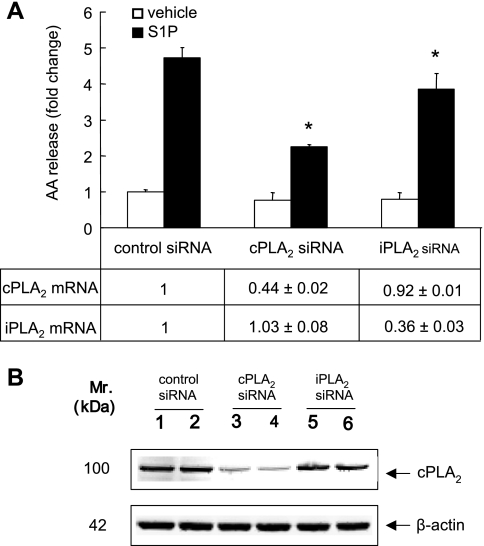
To determine whether cPLA2α is involved in S1P-induced AA release, we pretreated A549 cells with the cPLA2 inhibitors, MAFP or pyrrolidine derivative (1, 28, 30), and measured their effects on S1P-induced AA release. As shown in Fig. 2A, MAFP inhibited S1P-induced AA release in a dose-dependent manner (46 ± 5.3% and 72 ± 4.1% inhibition for 10 μM and 25 μM MAFP pretreatment, respectively). Since MAFP has been shown to be an iPLA2 inhibitor in vitro with an IC50 of 0.5 μM (23), we applied a specific inhibitor of cPLA2α, pyrrolidine derivative, to our experiments. As shown in Fig. 2B, pyrrolidine derivative inhibited S1P-induced AA release in a dose-dependent manner and nearly abolished the AA release at a concentration of 5 μM. To examine the role of iPLA2 in this event, we tested the effect of the iPLA2 inhibitor, BEL, on S1P-induced AA release. At the concentrations of 2 and 5 μM, BEL partially inhibited S1P-induced AA release of 14.2 ± 6.8% and 18.4 ± 6.3%, respectively (Fig. 2C). These are the concentrations that fully inhibited iPLA2 activity in a previous report (44). A higher concentration of BEL (50 μM) completely inhibited S1P-induced calcium flux (data not shown). To further determine the contributions of cPLA2α and iPLA2 in S1P-induced AA release, we knocked down the expression of cPLA2α or iPLA2 with siRNA. After 48 h of treatment with siRNAs, A549 cells expressed lower levels of cPLA2α or iPLA2 when examined by real-time PCR using TaqMan real-time analysis or immunoblotting using anti-cPLA2α antibody (Fig. 3, A, bottom, and B). As shown in Fig. 3A, top, compared with the cells transfected with control siRNA, the AA release induced by S1P was suppressed by 57.5 ± 1.2% and 16.2 ± 8.2% in cPLA2α and iPLA2 siRNA treated cells, respectively. We also determined the incorporation rate of 3H-AA labeled in A549 cells and found that the total cellular radioactivity is constant across the cells treated with cPLA2α, iPLA2, and control siRNA (data not shown). These data provide evidence that cPLA2α is a major but not the only phospholipase A2 activated in S1P-induced AA mobilization in A549 cells.
Fig. 2.
The inhibitory effects of MAFP, pyrrolidine derivative, and bromoenol lactone (BEL) on S1P-induced AA release. Cells labeled with 3H-AA were treated with MAFP (A), pyrrolidine derivative (B), or BEL (C) at the indicated concentrations for 30 min before 500 nM S1P stimulation. Means ± SD; n = 6. *P < 0.05 compared with control cells treated with S1P by Student's t-test.
Fig. 3.
The effect of downregulation of cytosolic phospholipase A2α (cPLA2α) or iPLA2 on S1P-induced AA release. Downregulation of cPLA2α or iPLA2 by small interfering RNAs (siRNAs) was performed as described in materials and methods, and cells were stimulated with 500 nM S1P for 10 min. The AA release was represented as fold change compared with the vehicle-stimulated control cells (A, top). Forty-eight hours after siRNA transfection, cells were harvested, and cPLA2α and iPLA2 mRNA expression levels were analyzed by real-time PCR TaqMan analysis (A, bottom) and Western blot analysis (B). The data represent the means ± SD of 6 samples from a single experiment representative of 3 independent experiments, *P < 0.01 by Student's t-test comparing S1P-treated knockdown cells and control siRNA-treated cells. Western blot analysis was consistent across 3 independent experiments. Real-time PCRs were done in triplicate in 2 independent experiments.
S1P-induced cPLA2α activation is calcium dependent.
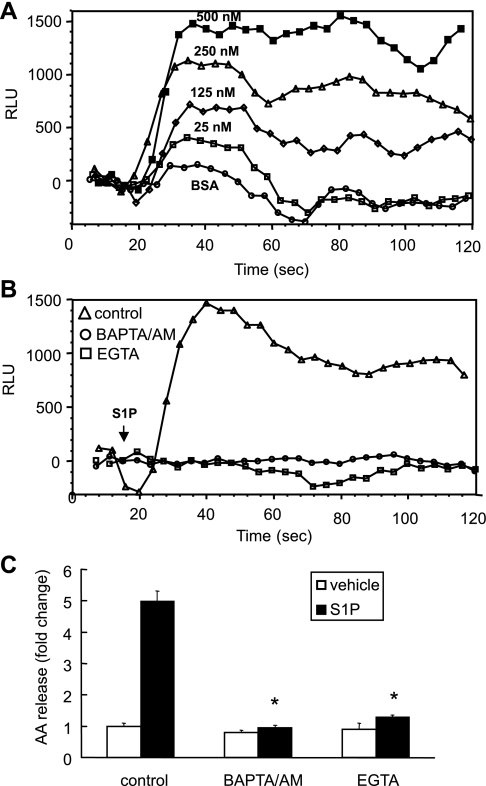
A submicromolar calcium concentration is essential for cPLA2α activation (11). To understand the contribution of [Ca2+]i in S1P-induced cPLA2 activation, we examined the ability of S1P to mobilize calcium in A549 cells. We found that S1P induced a transient and concentration-dependent increase in [Ca2+]i (Fig. 4A). Our data showed that the effective concentrations of S1P to mobilize calcium paralleled the concentrations of S1P-induced AA release, 25 nM at the onset and 500 nM at the maximum (Figs. 4A and 1B). To determine whether the intra- or extracellular calcium is crucial for S1P-induced calcium flux to activate cPLA2 to release AA, we pretreated cells with EGTA or BAPTA-AM (extracellular and intracellular calcium chelators, respectively) before S1P treatment and measured their effects on both S1P-induced calcium flux and AA release. As shown in Fig. 4B, S1P-elicited [Ca2+]i was totally suppressed in the cells treated with 5 mM EGTA or 30 μM BAPTA-AM. Both treatments also completely abolished the S1P-induced AA release (Fig. 4C). These findings provide evidence that S1P-induced calcium mobilization contributes to cPLA2α activation and AA release. Furthermore, both intra- and extracellular calcium are essential for this event.
Fig. 4.
The effects of EGTA and BAPTA-AM on S1P-induced calcium flux and AA release in A549 cells. Cells plated on 96-well plates were stimulated with different concentrations of S1P as indicated. The calcium flux was measured at a FlexStation and presented as relative light units (RLU) (A). Cells were pretreated with EGTA (5 mM) or BAPT-AM (30 μM) for 30 min and stimulated with 500 nM S1P to measure the calcium flux (B). Following 3H-AA labeling, cells were pretreated with EGTA (5 mM) and BAPTA-AM (30 μM) for 30 min and then stimulated with 500 nM S1P or vehicle for 10 min to measure the AA release. The AA release was represented as fold change compared with the vehicle-stimulated control cells (C). The data represent the means ± SD of 6 samples from a single experiment representative of 3 independent experiments, *P < 0.0001 compared with control cells treated with S1P by Student's t-test. Calcium flux measurements gave similar results from 3 independent experiments.
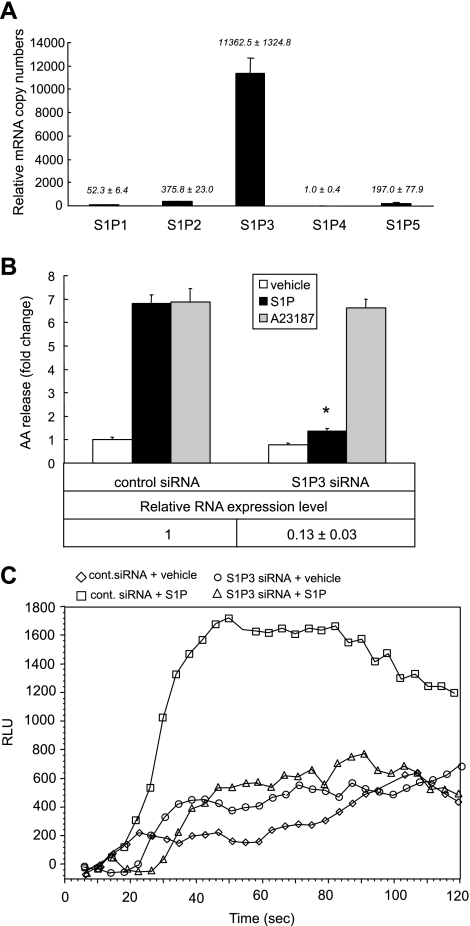
S1P3 mediates the S1P-induced cPLA2α activation.
S1P bound to G protein-coupled receptors (GPCR) on the cell surface mediates intracellular signals that regulate cell migration, proliferation, survival, and morphogenesis (45). To determine the role of S1P receptors in S1P-induced cPLA2α activation and AA release, we first performed real-time PCR to examine the expression patterns of five S1P receptors, S1P1-5 (endothelial differentiation gene: EDG 1, 5, 3, 6, and 8), which are receptors coupled to S1P signaling. We found that S1P3/EDG3 is the major receptor expressed in A549 cells (Fig. 5A). To determine the role of S1P3 in S1P-induced cPLA2α activation and AA release, we used siRNA to knockdown gene expression of S1P3. S1P-induced AA release was significantly decreased in S1P3 knockdown cells (Fig. 5B). We verified the decreased expression level of S1P3 by real-time PCR using TaqMan real-time analysis. S1P3 gene expression in S1P3 siRNA-treated cells was ∼13% of that in control siRNA-treated cells (Fig. 5B, bottom). The S1P3 downregulation did not change the potency of calcium ionophore A23187 (100 nM)-induced AA release (Fig. 5B), suggesting that the S1P3 siRNA transfection does not change the intracellular calcium pool.
Fig. 5.
S1P signals through S1P3 to induce calcium mobilization and AA release. Relative expression levels of S1P receptors (S1P1–5). Total RNA was extracted from cells after 48-h culture. Real-time PCR was performed to detect the relative expression levels of five S1P receptors compared with internal control of GAPDH gene expression (A). A549 cells were transfected with S1P3 siRNA or negative control sequence in 12-well plates for AA release, 96-well plates for calcium flux assay, and 6-well plates for real-time PCR analysis. Cells were treated with S1P (500 nM), A23187 (100 nM), or vehicle for 10 min. The AA release is presented as fold change compared with the vehicle-stimulated control cells (B, top). The downregulation of S1P3 is presented as fold change compared with negative control sequence transfected cells by real-time PCR (B, bottom). The calcium flux assay was performed in S1P3 siRNA-treated or control siRNA-treated cells in 96-well plates, with and without stimulation with 500 nM S1P (C, □: control siRNA-treated cells with S1P; ◊: control siRNA-treated cells with vehicle; ○: S1P3 siRNA-treated cells with vehicle; and ▵: S1P3 siRNA-treated cells with S1P). The AA release data represent the means ± SD of 6 samples from a single experiment representative of 3 independent experiments. *P < 0.0001 compared with control cells treated with S1P by Student's t-test. Real-time PCRs were done in triplicate in 2 independent experiments.
To dissect the signaling pathways mediated by S1P3 that lead to cPLA2α activation, we transfected S1P3 siRNA or control siRNA to knockdown S1P3 expression in A549 cells. We then analyzed the siRNA-treated cells by examining S1P-induced calcium flux. A significant calcium flux impairment was found in S1P3 downregulated cells (Fig. 5C) suggesting that calcium ion elevation caused by S1P stimulation plays a crucial role in S1P-induced cPLA2α activation that leads to AA release.
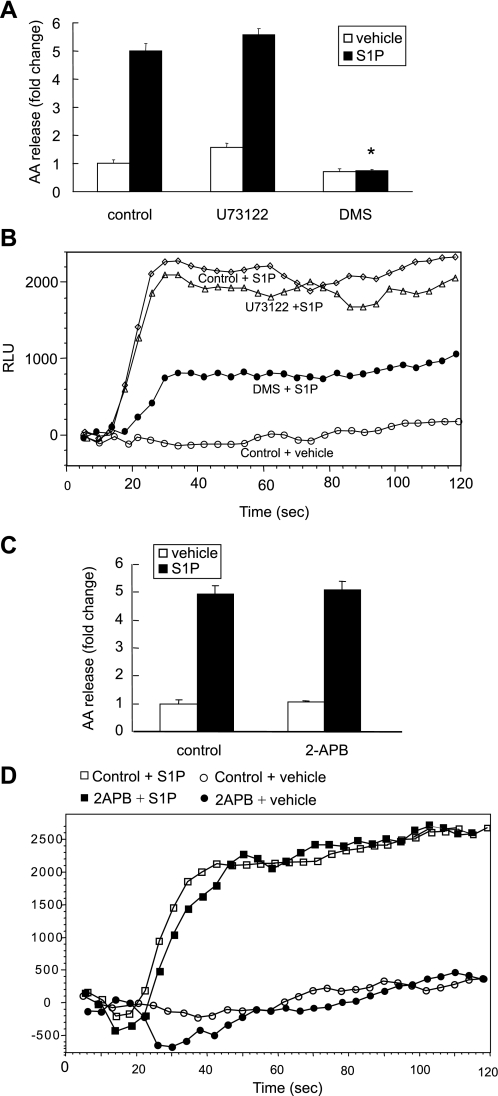
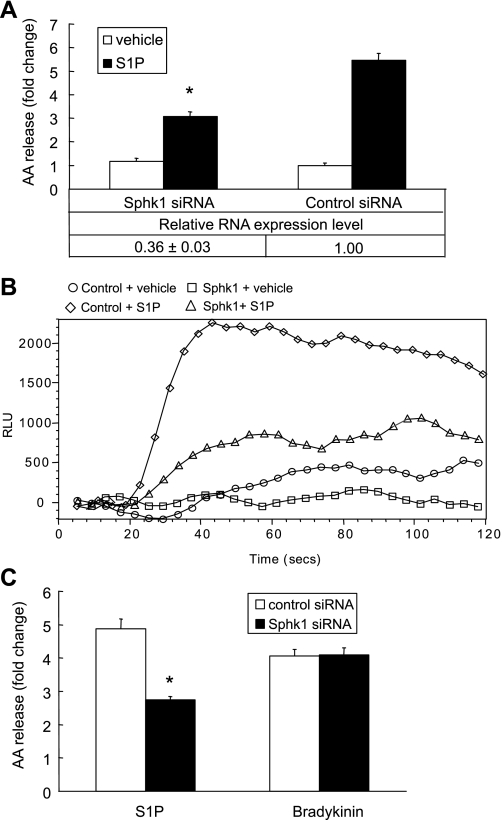
S1P-induced calcium flux is sphingosine kinase dependent.
The calcium mobilization induced by S1P can be regulated by the PLC/IP3 pathway or by endogenous S1P production (4). Since our previous results demonstrated that S1P-induced calcium flux contributed to cPLA2α activation, we further examined whether the IP3/PLC calcium release pathway or endogenous S1P formation is involved in the S1P-induced cPLA2α activation. We used the PLC inhibitor U73122, the IP3 receptor inhibitor 2-APB, and the Sphk inhibitor DMS to test their effects on S1P-induced calcium flux and AA release. Cells pretreated with the PLC inhibitor U73122 (5 μM) demonstrated no inhibition of calcium flux or AA release (Fig. 6, A and B). Treatment with the IP3 receptor inhibitor, 2-APB (25 μM), resulted in no inhibition of the AA release and calcium flux induced by S1P (Fig. 6, C and D). On the other hand, treatment with the sphingosine kinase inhibitor, DMS (20 μM), resulted in a complete suppression of S1P-induced AA release (Fig. 6A) and a substantial inhibition in S1P-induced calcium flux (Fig. 6B). To further verify the contribution of Sphk1 to S1P-induced AA release, we transfected A549 cells with Sphk1 siRNA for 48 h and examined the effects on S1P-induced AA release and calcium flux. We found that the Sphk1 siRNA transfected cells showed a 40.1 ± 3.0% decrease in S1P-induced AA release (Fig. 7A) and a significant decrease in calcium flux in response to S1P (Fig. 7B). We further tested the effect of Sphk1 downregulation on bradykinin-induced AA release. As shown in Fig. 7C, the downregulation of Sphk1 had no effect on bradykinin-induced AA release. These findings demonstrate that the calcium mobilization induced by S1P was independent of the U73122-sensitive/PLC pathway. Therefore, new formation of S1P, which was converted from sphingosine by sphingosine kinase (Sphk1), played a key role in S1P-induced calcium mobilization that leads to cPLA2α activation and AA release. We tested whether the S1P-induced calcium mobilization is related to LPP-1 using its specific inhibitor, XY-14 (54). We found that XY-14 (10 μM) pretreatment did not have an effect on S1P-induced AA release and calcium mobilization (data not shown). Based on our data, we suggest that extracellular S1P-induced formation of intracellular S1P might serve as a second messenger to induce calcium flux that contributes to cPLA2α activation and AA release.
Fig. 6.
Effects of inhibitors U73122, 2-APB, and DMS on S1P-induced AA release and calcium mobilization in A549 cells. Cells were seeded in 12-well plates for the AA release assay and 96-well plates for the calcium flux assay. Cells were pretreated with the Sphk1 inhibitor DMS (20 μM) for 30 min or U73122 (5 μM) or 2-APB (25 μM) for 5 min, and AA release was measured (A and C). The same inhibitory pretreatment was done before S1P stimulation for calcium flux measurement (B and D). The AA release data represent the means ± SD of 6 samples from a single experiment representative of 3 independent experiments, *P < 0.0001 compared with control cells treated with S1P by Student's t-test. Calcium flux studies gave similar results across 3 independent experiments.
Fig. 7.
Effects of Sphk1 downregulation on S1P and bradykinin-induced AA release or on S1P-induced calcium mobilization. Cells were seeded in 12-well plates for the AA release assay, 96-well plates for the calcium flux assay, and 6-well plates for real-time PCR after siRNA transfection. Cells were transfected with Sphk1 siRNA or negative control sequence for 48 h, and then the AA release (A, top) and calcium flux (B) were measured. The Sphk1 siRNA-treated cells were also stimulated with 100 nM bradykinin (C). Downregulation of Sphk1 by siRNA was confirmed using real-time PCR and presented as a fold change compared with negative control sequence transfected cells (A, bottom). AA release was presented as fold change compared with the vehicle-treated control cells. The AA release data represent the means ± SD of 6 samples from a single experiment representative of 3 independent experiments, *P < 0.001 by Student's t-test compared with control siRNA-treated cells. Real-time PCRs were done in triplicate in 2 independent experiments. Calcium flux studies gave similar results across 3 independent experiments.
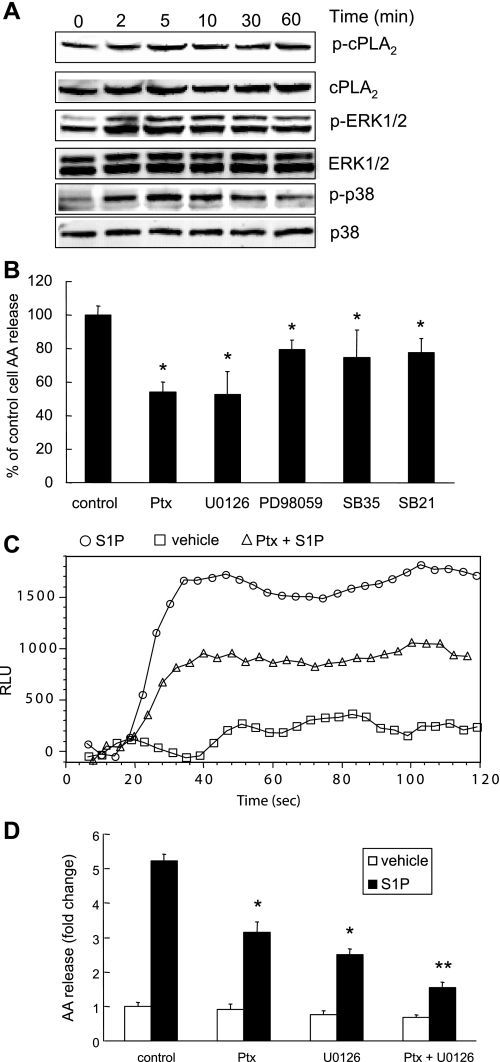
S1P-induced cPLA2α activation is partially dependent on MAP kinase activation.
To confirm whether S1P-induced MAP kinase activation contributed to S1P-induced cPLA2α activation, MAP kinase specific inhibitors were used. We first showed that S1P-induced MAP kinase (ERK1/2 and p38) activation is an early event. As shown in Fig. 8A, S1P induced ERK1/2 and p38 phosphorylation over 2–10 min. Inhibitors of p38 kinase, SB-203580 (10 μM), SB-202190 (3 μM), PD-98059 (10 μM, inhibitor of ERK1/2), and U0126 (10 μM, MEK1/2 inhibitor) were added to cells before S1P or vehicle (control) stimulation. Treatment with a variety of MAP kinase inhibitors resulted in a partial inhibition of S1P-induced AA release. U0126 treatment resulted in a more potent inhibitory effect than PD-98059 did on S1P-induced AA release. Both SB-203580 and SB-202190 had similar inhibitory effects on S1P-induced AA release (Fig. 7B). These findings suggested that S1P-induced cPLA2α activation is partially mediated by MAP kinases, p38 and ERK1/2, activation. S1P-induced AA release was mediated in part by Gi because its action was partially inhibited by pretreatment of the cells with 100 ng/ml of Ptx (Fig. 7B). Since Ptx pretreatment had no inhibitory effects on S1P-induced p38 and ERK1/2 phosphorylation (data not shown), we then examined the effect of Ptx on S1P-induced calcium flux. As shown in Fig. 8C, Ptx treatment decreased S1P-induced calcium flux. As shown in Fig. 8D, there was an additive effect of Ptx and U0126 on S1P-induced AA release suggesting that p38 and ERK1/2 MAP kinase pathways and Ptx-sensitive Gi-coupled signaling contribute to S1P-induced AA release independently.
Fig. 8.
Pertussis toxin (Ptx) and MAP kinase inhibitors decrease S1P-induced AA release or calcium mobilization in A549 cells. The time course of S1P-induced cPLA2α, ERK, and p38 phosphorylation was shown in A. Following 3H-AA labeling, cells were pretreated with Ptx (100 ng/ml) for 18 h, or U0126 (10 μM), PD-98059 (10 μM), SB-203580/SB35 (10 μM), or SB-202190/SB21 (3 μM) for 30 min followed by stimulation with 500 nM S1P for 10 min. The AA release assay is represented as percentage of control cells AA release (B). The calcium flux assay was performed in 96-well plates, and cells were stimulated with 500 nM S1P (C, ○: S1P; □: vehicle; ▵: Ptx-treated cells with S1P). The combined effect of Ptx and U0126 on S1P-induced AA release is represented in D. The AA release data represent the means ± SD from 3 independent experiments, *P < 0.001 by Student's t-test compared with control cells stimulated with S1P. **P < 0.001 by Student's t-test in comparison of U0126/Ptx-treated cells with U0126 or Ptx-treated cells. The calcium flux analysis gave similar results across 3 independent experiments.
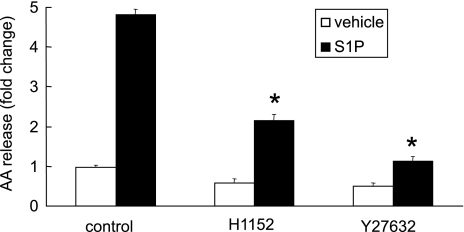
Rho-associated kinase is essential for S1P-induced cPLA2α activation.
To examine whether S1P3 receptor coupled to a G12/13/Rho-associated kinase (ROCK) contributes to S1P-induced cPLA2α activation, we treated cells with two ROCK inhibitors, H1152 and Y27632, before S1P stimulation. Both ROCK inhibitors significantly inhibited the S1P-induced AA release (Fig. 9). We then examined the effect of H1152 and Y27632 on S1P-induced MAPK activation and calcium flux. We found that these two inhibitors had no inhibitory effect on MAPK activation or calcium flux induced by S1P suggesting that ROCK might contribute to cPLA2α activation by a separate downstream mechanism. Therefore, S1P through S1P3 activates cPLA2α via a flux of intracellular free calcium and MAPK and ROCK-dependent pathways.
Fig. 9.
The effects of Y27632 and H1152 on S1P-induced AA release in A549 cells. Cells labeled with 3H-AA were pretreated with Y27632 (10 μM) or H1152 (3 μM) for 30 min and then stimulated with 500 nM S1P for 10 min. The AA release is presented as fold change compared with the vehicle-stimulated control cells. Means ± SD; n = 6. *P < 0.0001 compared with control cells treated with S1P by Student's t-test.
DISCUSSION
The airway epithelium plays a crucial role in initiating and augmenting pulmonary host defense. Cells lacking cPLA2α are generally devoid of agonist-receptor-activated eicosanoid synthesis. Control of cPLA2α activation in lung epithelium may provide an opportunity for controlling pulmonary inflammatory processes. S1P is a naturally generated molecule present in serum and biological fluids and a key mediator in immune cell trafficking. It is elevated in the lungs of asthmatic patients and regulates pulmonary epithelial permeability and mast cell functions (13, 19, 29, 31). An understanding of the effect of S1P on cPLA2α activation in epithelial cells will provide a better understanding of inflammatory events mediated by cPLA2α and S1P. Here, we have demonstrated that S1P induces cPLA2α activation and AA release in lung epithelial cells acting at least through the S1P3 receptor and downstream signaling pathways.
S1P is stored and released from platelets upon their activation, but can also be synthesized in a wide variety of cell types in response to extracellular stimuli, such as growth factors and cytokines. The plasma level of S1P has been reported to be 190–350 nM (38, 52). S1P induces A549 cells to release AA in a dose-dependent manner. It is an early phase event with onset at 5 min (Fig. 1A), suggesting that S1P-induced AA release is a specific event that could be achieved by signal transduction pathways mediated by S1P receptors. Agonist-induced AA release can be achieved by activating several pathways, such as PLC/DAG lipase, PLD/DAG lipase, and PLA2. Previous studies suggested that the PLC/DAG or PLD/DAG pathway is not likely involved in S1P-induced AA release (50). In our study, three lines of evidence suggest that cPLA2α activation mediates S1P-induced AA release. First, S1P-induced AA release is inhibited by the cPLA2 inhibitors MAFP and pyrrolidine derivative. Second, downregulation of cPLA2α expression by siRNA inhibited S1P-induced AA release significantly. Third, calcium chelators, EGTA and BAPTA-AM, abolished S1P-induced AA release.
It has been reported that calcium-independent PLA2 (iPLA2) contributes to S1P-induced AA release because of an inhibitory effect of BEL at concentrations of 10, 30, and 60 μM in A549 cells (50). BEL is a potent inhibitor of iPLA2 with an IC50 of 60 nM and exhibits 1,000-fold higher selectivity for calcium-independent PLA2 vs. calcium-dependent PLA2 (1). Our results showed partial inhibitory effects of BEL in the concentration of 2 and 5 μM (Fig. 2C), which are the concentrations that fully inhibited iPLA2 activity in a previous report (44). Higher doses of BEL might have additional effects. Interestingly, we found an inhibitory effect of BEL on S1P-induced calcium flux at a concentration of 50 μM. Additional evidence to support a primary role of cPLA2α in S1P-induced AA release is that downregulation of cPLA2α is more effective than downregulation of iPLA2 in inhibiting S1P-induced AA release. We found that the S1P-induced AA release was completely abolished when the cells were pretreated with EGTA (5 mM) and BAPTA-AM (30 μM), which chelated the extracellular and intracellular calcium, respectively (Fig. 4B). A major difference between cPLA2α and iPLA2 is a lack of calcium requirement for iPLA2 enzyme activity (27). Although iPLA2 partially contributed to S1P-induced AA release, our data suggest that cPLA2α is the major phospholipase contributing to S1P-induced AA release.
In addition to S1P, ceramide 1-phosphate (C1P) is another phosphorylated sphingolipid metabolite that has emerged as a potent bioactive agent. S1P and C1P act synergistically in the induction of prostaglandin E2 (PGE2) formation with two independent but coordinate pathways (7, 36, 37). Some studies have addressed the biological and biochemical effects of C1P on cPLA2α activation (34, 35, 46). C1P is a potent lipid mediator stimulating AA release over a period of 2–24 h. These studies also demonstrated that C1P is a direct activator of cPLA2α by positive allosteric activation. In our study, S1P activated cPLA2α and AA release very quickly, suggesting an early phase signaling event (within 5 min). Thus, S1P signaling to cPLA2α activation and AA release seems to be differently regulated compared with C1P-mediated cPLA2α activation. Our findings might also explain the coordinated effects on PGE2 induction by S1P and C1P. Collectively, these findings imply that different bioactive lipids (S1P and C1P) might regulate different phases of cPLA2α activation, and they both are potential targets to control inflammatory responses.
The S1P3 is the receptor most abundantly expressed in A549 cells (Fig. 5A). This is in agreement with a prior study reporting that S1P3 mRNA is abundantly expressed in the pulmonary epithelium (13). Downregulation of S1P3 inhibited S1P-induced calcium flux (Fig. 5C) and decreased S1P-induced AA release (more than 80%) (Fig. 5B) suggesting that S1P3 is the major receptor mediating the S1P signaling for calcium mobilization and activation of cPLA2α. We have not excluded the possible participation of other S1P receptors in this event. Previous studies have reported that S1P3 might signal via Ptx-sensitive, Gi-coupled pathways, PLC-dependent Gq-mediated events, as well as Ptx-independent transduction steps, i.e., activation of Rho small GTP-binding proteins (39, 40). In this study, we found that the calcium flux elicited by S1P seems to dominate the signaling pathway to modify the activity of cPLA2α. The inhibitory effects of Ptx on S1P-induced AA release appear to be due to its inhibition on calcium flux (Fig. 8C). In general, the calcium mobilization induced by S1P can be regulated by the PLC/IP3 pathway or by endogenous S1P production (4). The PLC inhibitor, U73122, had no inhibitory effects on both S1P-induced AA release and calcium flux, suggesting that the downstream signaling of S1P3 for calcium flux is unlikely to be related to the Gq-coupled PLC pathway. Independent of the known G protein-coupled signaling, the sphingosine kinase inhibitor, DMS, almost completely abolished S1P-induced AA release and significantly inhibited S1P-induced calcium flux. Downregulation of Sphk1 by siRNA resulted in significantly decreased S1P-induced AA release and calcium flux but not in bradykinin-induced AA release. Bradykinin is a known agonist to stimulate the AA release in A549 cells, and its action is likely via G protein-coupled receptors to activate a Gi- or Gq-mediated signaling pathways (21). These data suggested that a S1P/Sphk1 pathway might contribute to intracellular generation of S1P to serve as a second messenger to induce calcium flux and AA release. Furthermore, the mechanism of Sphk1 action in S1P-induced calcium flux and AA release in A549 cells is independent of LPP-1, which was shown in HUVEC to enhance intracellular S1P generation (54). However, the mechanism of extracellular S1P-induced intracellular S1P formation in the epithelium warrants further study. Together, the signaling pathway induced by S1P to cause cPLA2α activation is associated with S1P3 coupled to Gi- and Sphk1-mediated calcium flux. The study of a variety of kinase inhibitors, U0126-MEK1/2, PD-98059-ERK1/2, and SB-203580 and SB-202190-p38, suggested that S1P-induced cPLA2α activation is associated with MEK1/2, ERK1/2, and p38 activation.
The small GTPase, Rho, is an important downstream signaling molecule after S1P receptor/G12/13 activation. The pretreatment of ROCK-specific inhibitors, H1152 and Y27632, resulted in decreased AA release induced by S1P (Fig. 9). The treatment of cells with H1152 and Y27632 did not change calcium flux or MAPK activation induced by S1P, suggesting that Rho-associated kinase may alter S1P-induced cPLA2α activation downstream of these events.
In conclusion, we have shown that S1P activates cPLA2α mainly through the elevation of intracellular free calcium via S1P3 to release AA. The calcium flux induced by S1P is PLC pathway independent and is associated with sphingosine kinase activity and Gi-coupled events. Downstream of G12/13-coupled events, Rho kinase activation is involved in cPLA2α translocation induced by S1P. The contribution of these mechanisms to cPLA2α activation in epithelial cells and the potential role of S1P3 blockade therapeutically are worthy of further study.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ackermann EJ, Conde-Frieboes K, Dennis EA. Inhibition of macrophage Ca2+-independent phospholipase A2 by bromoenol lactone and trifluoromethyl ketones. J Biol Chem 270: 445–450, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Ammit AJ, Hastie AT, Edsall LC, Hoffman RK, Amrani Y, Krymskaya VP, Kane SA, Peters SP, Penn RB, Spiegel S, Panettieri RA Jr. Sphingosine 1-phosphate modulates human airway smooth muscle cell functions that promote inflammation and airway remodeling in asthma. FASEB J 15: 1212–1214, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Billich A, Bornancin F, Mechtcheriakova D, Natt F, Huesken D, Baumruker T. Basal and induced sphingosine kinase 1 activity in A549 carcinoma cells: function in cell survival and IL-1beta and TNF-alpha induced production of inflammatory mediators. Cell Signal 17: 1203–1217, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Blom T, Slotte JP, Pitson SM, Tornquist K. Enhancement of intracellular sphingosine-1-phosphate production by inositol 1,4,5-trisphosphate-evoked calcium mobilisation in HEK-293 cells: endogenous sphingosine-1-phosphate as a modulator of the calcium response. Cell Signal 17: 827–836, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Bonventre J Cytosolic phospholipase A2alpha reigns supreme in arthritis and bone resorption. Trends Immunol 25: 116–119, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Bonventre JV, Huang Z, Taheri MR, O'Leary E, Li E, Moskowitz MA, Sapirstein A. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature 390: 622–625, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Chalfant CE, Spiegel S. Sphingosine 1-phosphate and ceramide 1-phosphate: expanding roles in cell signaling. J Cell Sci 118: 4605–4612, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Clark JD, Lin LL, Kriz RW, Ramesha CS, Sultzman LA, Lin AY, Milona N, Knopf JL. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca2+-dependent translocation domain with homology to PKC and GAP. Cell 65: 1043–1051, 1991. [DOI] [PubMed] [Google Scholar]
- 9.Cummings RJ, Parinandi NL, Zaiman A, Wang L, Usatyuk PV, Garcia JG, Natarajan V. Phospholipase D activation by sphingosine 1-phosphate regulates interleukin-8 secretion in human bronchial epithelial cells. J Biol Chem 277: 30227–30235, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Dennis EA The growing phospholipase A2 superfamily of signal transduction enzymes. Trends Biochem Sci 22: 1–2, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Dessen A, Tang J, Schmidt H, Stahl M, Clark JD, Seehra J, Somers WS. Crystal structure of human cytosolic phospholipase A2 reveals a novel topology and catalytic mechanism. Cell 97: 349–360, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Gijon MA, Spencer DM, Leslie CC. Recent advances in the regulation of cytosolic phospholipase A(2). Adv Enzyme Regul 40: 255–268, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Gon Y, Wood MR, Kiosses WB, Jo E, Sanna MG, Chun J, Rosen H. S1P3 receptor-induced reorganization of epithelial tight junctions compromises lung barrier integrity and is potentiated by TNF. Proc Natl Acad Sci USA 102: 9270–9275, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Hiller G, Sundler R. Activation of arachidonate release and cytosolic phospholipase A2 via extracellular signal-regulated kinase and p38 mitogen-activated protein kinase in macrophages stimulated by bacteria or zymosan. Cell Signal 11: 863–869, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Hirabayashi T, Murayama T, Shimizu T. Regulatory mechanism and physiological role of cytosolic phospholipase A2. Biol Pharm Bull 27: 1168–1173, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Hla T, Lee MJ, Ancellin N, Liu CH, Thangada S, Thompson BD, Kluk M. Sphingosine-1-phosphate: extracellular mediator or intracellular second messenger? Biochem Pharmacol 58: 201–207, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Hla T, Lee MJ, Ancellin N, Paik JH, Kluk MJ. Lysophospholipids-receptor revelations. Science 294: 1875–1878, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, Rivera J, Milstien S, Spiegel S. Transactivation of sphingosine-1-phosphate receptors by FcepsilonRI triggering is required for normal mast cell degranulation and chemotaxis. J Exp Med 199: 959–970, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jolly PS, Rosenfeldt HM, Milstien S, Spiegel S. The roles of sphingosine-1-phosphate in asthma. Mol Immunol 38: 1239–1245, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, Volpi M, Sha'afi RI, Hla T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 99: 301–312, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Leeb-Lundberg LM Bradykinin specificity and signaling at GPR100 and B2 kinin receptors. Br J Pharmacol 143: 931–932, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin LL, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ. cPLA2 is phosphorylated and activated by MAP kinase. Cell 72: 269–278, 1993. [DOI] [PubMed] [Google Scholar]
- 23.Lio YC, Reynolds LJ, Balsinde J, Dennis EA. Irreversible inhibition of Ca2+-independent phospholipase A2 by methyl arachidonyl fluorophosphonate. Biochim Biophys Acta 1302: 55–60, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Chakravarty D, Maceyka M, Milstien S, Spiegel S. Sphingosine kinases: a novel family of lipid kinases. Prog Nucleic Acid Res Mol Biol 71: 493–511, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Mattie M, Brooker G, Spiegel S. Sphingosine-1-phosphate, a putative second messenger, mobilizes calcium from internal stores via an inositol trisphosphate-independent pathway. J Biol Chem 269: 3181–3188, 1994. [PubMed] [Google Scholar]
- 26.McVerry BJ, Garcia JG. In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights. Cell Signal 17: 131–139, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Murakami M, Kudo I. Phospholipase A2. J Biochem 131: 285–292, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Ni Z, Okeley NM, Smart BP, Gelb MH. Intracellular actions of group IIA secreted phospholipase A2 and group IVA cytosolic phospholipase A2 contribute to arachidonic acid release and prostaglandin production in rat gastric mucosal cells and transfected human embryonic kidney cells. J Biol Chem 281: 16245–16255, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olivera A, Mizugishi K, Tikhonova A, Ciaccia L, Odom S, Proia RL, Rivera J. The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity 26: 287–297, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Ono T, Yamada K, Chikazawa Y, Ueno M, Nakamoto S, Okuno T, Seno K. Characterization of a novel inhibitor of cytosolic phospholipase A2alpha, pyrrophenone. Biochem J 363: 727–735, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oz-Arslan D, Ruscher W, Myrtek D, Ziemer M, Jin Y, Damaj BB, Sorichter S, Idzko M, Norgauer J, Maghazachi AA. IL-6 and IL-8 release is mediated via multiple signaling pathways after stimulating dendritic cells with lysophospholipids. J Leukoc Biol 80: 287–297, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Pawliczak R, Han C, Huang XL, Demetris AJ, Shelhamer JH, Wu T. 85-kDa cytosolic phospholipase A2 mediates peroxisome proliferator-activated receptor gamma activation in human lung epithelial cells. J Biol Chem 277: 33153–33163, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Pawliczak R, Huang XL, Nanavaty UB, Lawrence M, Madara P, Shelhamer JH. Oxidative stress induces arachidonate release from human lung cells through the epithelial growth factor receptor pathway. Am J Respir Cell Mol Biol 27: 722–731, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Pettus BJ, Bielawska A, Spiegel S, Roddy P, Hannun YA, Chalfant CE. Ceramide kinase mediates cytokine- and calcium ionophore-induced arachidonic acid release. J Biol Chem 278: 38206–38213, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Pettus BJ, Bielawska A, Subramanian P, Wijesinghe DS, Maceyka M, Leslie CC, Evans JH, Freiberg J, Roddy P, Hannun YA, Chalfant CE. Ceramide 1-phosphate is a direct activator of cytosolic phospholipase A2. J Biol Chem 279: 11320–11326, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Pettus BJ, Bielawski J, Porcelli AM, Reames DL, Johnson KR, Morrow J, Chalfant CE, Obeid LM, Hannun YA. The sphingosine kinase 1/sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-alpha. FASEB J 17: 1411–1421, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Pettus BJ, Kitatani K, Chalfant CE, Taha TA, Kawamori T, Bielawski J, Obeid LM, Hannun YA. The coordination of prostaglandin E2 production by sphingosine-1-phosphate and ceramide-1-phosphate. Mol Pharmacol 68: 330–335, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Prescott SM A thematic series on phospholipases. J Biol Chem 272: 15043, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Pyne S, Pyne N. Sphingosine 1-phosphate signalling via the endothelial differentiation gene family of G-protein-coupled receptors. Pharmacol Ther 88: 115–131, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Pyne S, Pyne NJ. Sphingosine 1-phosphate signalling in mammalian cells. Biochem J 349: 385–402, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiu ZH, Gijon MA, de Carvalho MS, Spencer DM, Leslie CC. The role of calcium and phosphorylation of cytosolic phospholipase A2 in regulating arachidonic acid release in macrophages. J Biol Chem 273: 8203–8211, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Six DA, Dennis EA. Essential Ca2+-independent role of the group IVA cytosolic phospholipase A(2) C2 domain for interfacial activity. J Biol Chem 278: 23842–23850, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Six DA, Dennis EA. The expanding superfamily of phospholipase A(2) enzymes: classification and characterization. Biochim Biophys Acta 1488: 1–19, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Song Y, Wilkins P, Hu W, Murthy KS, Chen J, Lee Z, Oyesanya R, Wu J, Barbour SE, Fang X. Inhibition of calcium-independent phospholipase A2 suppresses proliferation and tumorigenicity of ovarian carcinoma cells. Biochem J 406: 427–436, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spiegel S, Milstien S. Exogenous and intracellularly generated sphingosine 1-phosphate can regulate cellular processes by divergent pathways. Biochem Soc Trans 31: 1216–1219, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Subramanian P, Stahelin RV, Szulc Z, Bielawska A, Cho W, Chalfant CE. Ceramide 1-phosphate acts as a positive allosteric activator of group IVA cytosolic phospholipase A2 alpha and enhances the interaction of the enzyme with phosphatidylcholine. J Biol Chem 280: 17601–17607, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Taha TA, Argraves KM, Obeid LM. Sphingosine-1-phosphate receptors: receptor specificity versus functional redundancy. Biochim Biophys Acta 1682: 48–55, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Tani M, Sano T, Ito M, Igarashi Y. Mechanisms of sphingosine and sphingosine 1-phosphate generation in human platelets. J Lipid Res 46: 2458–2467, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Uozumi N, Kume K, Nagase T, Nakatani N, Ishii S, Tashiro F, Komagata Y, Maki K, Ikuta K, Ouchi Y, Miyazaki J, Shimizu T. Role of cytosolic phospholipase A2 in allergic response and parturition. Nature 390: 618–622, 1997. [DOI] [PubMed] [Google Scholar]
- 50.Vasta V, Meacci E, Catarzi S, Donati C, Farnararo M, Bruni P. Sphingosine 1-phosphate induces arachidonic acid mobilization in A549 human lung adenocarcinoma cells. Biochim Biophys Acta 1483: 154–160, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Wu T, Han C, Shelhamer JH. Involvement of p38 and p42/44 MAP kinases and protein kinase C in the interferon-gamma and interleukin-1alpha-induced phosphorylation of 85-kDa cytosolic phospholipase A(2) in primary human bronchial epithelial cells. Cytokine 25: 11–20, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Yatomi Y Plasma sphingosine 1-phosphate metabolism and analysis. Biochim Biophys Acta 1780: 606–611, 2008. [DOI] [PubMed] [Google Scholar]
- 53.Zhang YH, Vasko MR, Nicol GD. Intracellular sphingosine 1-phosphate mediates the increased excitability produced by nerve growth factor in rat sensory neurons. J Physiol 575: 101–113, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao Y, Kalari SK, Usatyuk PV, Gorshkova I, He D, Watkins T, Brindley DN, Sun C, Bittman R, Garcia JG, Berdyshev EV, Natarajan V. Intracellular generation of sphingosine 1-phosphate in human lung endothelial cells: role of lipid phosphate phosphatase-1 and sphingosine kinase 1. J Biol Chem 282: 14165–14177, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou H, Das S, Murthy KS. Erk1/2- and p38 MAP kinase-dependent phosphorylation and activation of cPLA2 by m3 and m2 receptors. Am J Physiol Gastrointest Liver Physiol 284: G472–G480, 2003. [DOI] [PubMed] [Google Scholar]