Table 1.
Cross reactivity (%) of molinate and atrazine PHAIA
| Compound | Structure | Chemical hapten 19 | Clone 1M CSTWDTTGWC | Clone 2EM CRSHWDTWC | |
|---|---|---|---|---|---|
| molinate |
|
100 | 100 | 100 | |
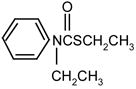
| thiobencarb |
|
1 | 0 | 0 | |
| butylate |
|
1 | 0 | 0 | |
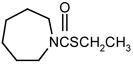
| EPTC |
|
1 | 5 | 15 | |
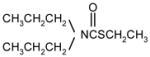
| cycloate |
|
1 | 9 | 0 | |
| pebulate |
|
4 | 7 | 13 | |
| Vernolate |
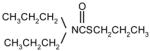
|
4 | 4 | 5 | |
| Compound | Structure | Chemical hapten 17 | Clone 13A CTPVRWFDMC | ||
|
| |||||
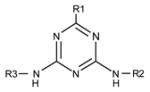
|
R1 | R2 | R3 | ||
| atrazine | Cl | C2H5 | CH(CH3)2 | 100 | 100 |
| simazine | Cl | C2H5 | C2H5 | 48 | 0 |
| propazine | Cl | CH(CH3)2 | CH(CH3)2 | 116 | 144 |
| cyanazine | Cl | C2H5 | C2N(CH3)2 | 91 | 0 |
| ametryn | SCH3 | C2H5 | CH(CH3)2 | 0 | 0 |
| simetryn | SCH3 | C2H5 | C2H5 | 0 | 0 |
| prometryn | SCH3 | CH(CH3)2 | CH(CH3)2 | 0 | 0 |
| terbutryn | SCH3 | C2H5 | C(CH3)3 | 0 | 0 |
All data are the mean of two independent experiments. A value of 0 means that there was no observable cross reactivity with the highest concentration tested 104 ng/ml
