Fig. 2.
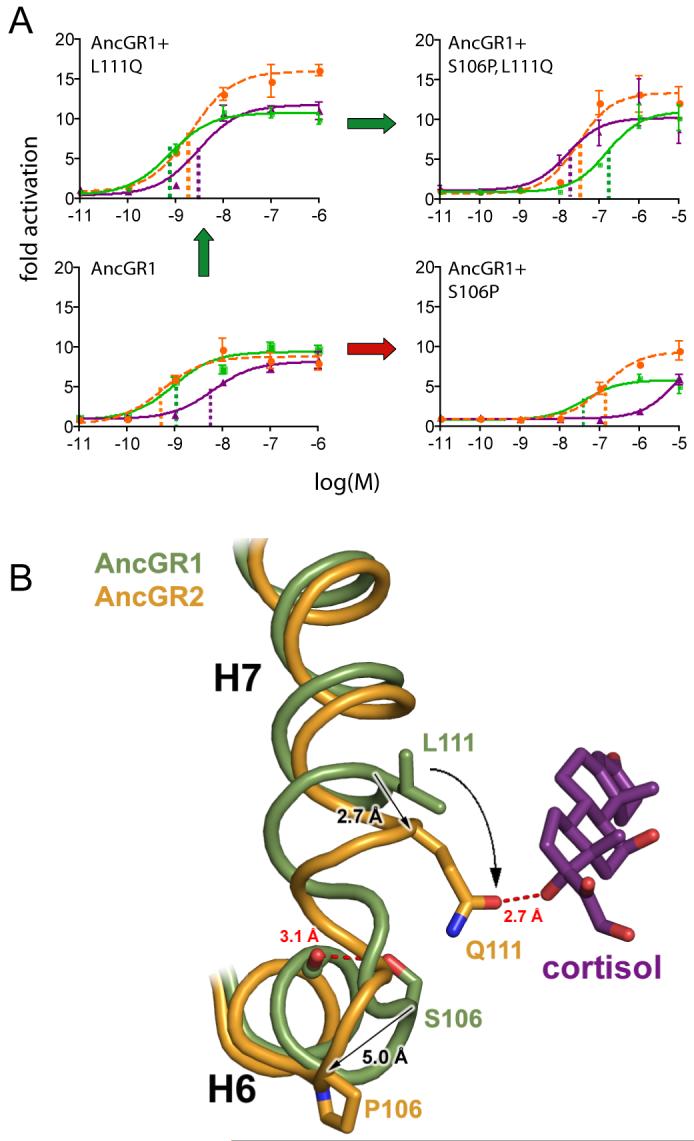
Mechanism for switching AncGR1’s ligand preference from aldosterone to cortisol. A) Effect of substitutions S106P and L111Q on the resurrected AncGR1’s response to hormones. Dashed lines indicate sensitivity to aldosterone (green), cortisol (purple), and DOC (orange) as the EC50 (concentration at which half-maximal reporter activation is achieved). Green arrow shows probable pathway through a functional intermediate; red arrow, intermediate with radically reduced sensitivity to all hormones. B) Structural change conferring new ligand specificity. Backbones of helices 6 and 7 from AncGR1 (green) and AncGR2 (yellow) in complex with cortisol are superimposed. Substitution S106P induces a kink in the interhelical loop of AncGR2, repositioning sites 106 and 111 (arrows). In this background, L111Q forms a new hydrogen bond with cortisol’s unique C17-hydroxyl (dotted red line).
