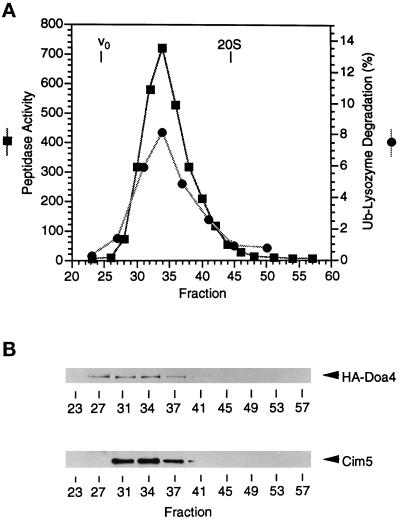
Figure 5.
Proteasome fractionation by Superose 6 gel filtration. (A) Peptidase activity and polyubiquitin(Ub)–[125I]-lysozyme degradation in fractions from a Superose 6 gel filtration column. The column was loaded with the pooled and concentrated 26S proteasome-containing Mono Q fractions (Figure 4). Ub–lysozyme degradation is reported as the percentage of total 125I radioactivity that was acid-soluble after a 45 min reaction with 50 μl of each fraction. The elution peak of 20S proteasomes (700 kDa) and the void volume, Vo (5 × 106 D, calibrated with blue dextran) are indicated. (B) Immunoblot analysis of HA-Doa4 and Cim5 proteins in the Superose 6 fractions.