Abstract
A precise understanding of neural circuits controlling lipid mobilization and thermogenesis remains to be determined. We have been studying the sympathetic nervous system (SNS) contributions to white adipose tissue (WAT) lipolysis largely in Siberian hamsters. Central melanocortins are implicated in the control of the sympathetic outflow to WAT, and, moreover, the melanocortin 4 receptors (MC4-R) appear to be principally involved. We previously found that acute third ventricular melanotan II (MTII; an MC3/4-R agonist) injections increase sympathetic drive (norepinephrine turnover) to interscapular brown adipose tissue (IBAT) and IBAT temperature. Here we tested whether MC4-R mRNA is expressed in IBAT SNS outflow neurons using in situ hybridization for the former and injections of the transneuronal viral retrograde tract tracer, pseudorabies virus (PRV) into IBAT, for the latter. Significant numbers of double-labeled cells for PRV and MC4-R mRNA were found across the neuroaxis (mean of all brain sites ∼60%), including the hypothalamic paraventricular nucleus (PVH; ∼80%). Acute parenchymal MTII microinjections into the PVH of awake, freely-moving hamsters, using doses below those able to increase IBAT temperature when injected into the third ventricle, increased IBAT temperature for as long as 4 h, as measured by temperature transponders implanted below the tissue. Collectively, these data add significant support to the view that central melanocortins are important in controlling IBAT thermogenesis via the SNS innervation of this tissue, likely through the MC4-Rs.
Keywords: Siberian hamsters, in situ hybridization, pseudorabies virus, tract tracing, melanocortins
a complete understanding of the neural circuits controlling the sympathetic nervous system (SNS) mobilization of lipid from white adipose tissue (WAT) and those triggering thermogenesis in brown adipose tissue (BAT) remain to be determined, although our knowledge has increased markedly in the past decade (for review, see Refs. 6 and 16). We have been studying the SNS contributions to the reversal of the naturally-occurring seasonal obesity of Siberian hamsters (Phodopus sungorus; for a review, see Ref. 5) in WAT, and to a lesser extent BAT (4, 11, 12, 18, 49). Of the many remaining unknown details of the SNS outflow circuitry to WAT and BAT is the identification of their neurochemical phenotype, although some progress has been made for both tissues (e.g., 8, 39, 48, 51, 53, 56). One of the neurochemicals strongly implicated in the sympathetic control of these adipose tissues is the melanocortins (for review, see Refs. 6 and 45). The most important members of the melanocortin family for the control of energy balance is α-melanocyte stimulating hormone (α-MSH), an agonist for melanocortin receptors (MCRs) derived from the posttranslational processing of the propeptide hormone proopiomelanocortin that is produced in the arcuate nucleus (Arc) and the nucleus of the solitary tract (Sol). Another important member of the melanocortin family for the control of energy balance is the agouti-related protein, also produced in the Arc and an inverse agonist for MCRs, (for a review, see Ref. 42). Of the known MCRs, the most important in the central control of energy balance appears to be the melanocortin 4 receptor (MC4-R), with the MC3-R receiving lesser support (for a review, see Ref. 19). Intracerebroventricularly administered melanotan II (MTII), a synthetic analog of α-MSH, triggers decreases in body fat of laboratory rats (43), which cannot be accounted for by the well-established MTII-induced inhibition of food intake alone (e.g., 24 and 27). Specifically, ad libitum-fed rats injected centrally with MTII exhibit a slight, but significant exaggerated body fat loss compared with their pair-fed counterparts (43), implicating both lipid mobilization and increases in thermogenesis that could occur via the SNS outflow to WAT and BAT. We recently reported that acute third ventricular injection of MTII increases the sympathetic drive (norepinephrine turnover) to both WAT and interscapular BAT (IBAT), as well as increasing circulating concentrations of glycerol and free fatty acids, the products of lipolysis, and IBAT temperature in Siberian hamsters (11). These data strongly implicate central melanocortin receptor stimulation for WAT lipolysis and are supported by the recent findings of others in laboratory rats (37) as well as adding to the importance of central melanocortins in BAT thermogenesis (e.g., 50, 56–58).
To describe the origins of the sympathetic outflow from brain to a variety of peripheral tissues, a transneuronal viral retrograde tract tracer, the pseudorabies virus (PRV), has been used in a number of species (for review, see Refs. 14 and 52). Because BAT only possess sympathetic (for a review, see Ref. 7) but not parasympathetic innervation [except for the mediastinal BAT depot and not IBAT investigated here (21)] and because of the retrograde-only directional spread of the Bartha's K PRV strain, the origins of the sympathetic outflow from brain to BAT have been established with this method (4, 13, 38, 39, 56). We previously utilized a strategy to define the SNS outflow circuits to WAT and the possible involvement of MCRs, by combining immunohistochemistry for PRV with in situ hybridization for MC4-R mRNA, thereby mapping the colocalization pattern of sympathetic outflow neurons to WAT possessing gene expression for this MCR (53). This approach will be used here to define the presence and extent of MC4-R mRNA on SNS outflow neurons to BAT.
Our functional work with MTII and norepinephrine turnover in BAT and WAT discussed above (11), as well as our success in colocalizing MC4-R mRNA with PRV-immunoreactive cells after virus injection into WAT (53) also discussed above, led us to conduct the present experiment. Therefore, in this study, we first asked, is MC4-R mRNA expressed in sympathetic outflow neurons to IBAT? Secondly we asked, does MTII injection into a site of high MC4-R mRNA and PRV colocalization (MC4-R mRNA + PRV), the hypothalamic paraventricular nucleus (PVH) implicated in the sympathetic control of IBAT thermogenesis (e.g., 20, 60), increase IBAT temperature in Siberian hamsters? To answer the first question we inoculated IBAT unilaterally with PRV and then processed the brains for in situ hybridization and immunohistochemistry to test for MC4-R + PRV colocalization. To answer the second question, we microinjected MTII unilaterally into the PVH, an area with exceptionally high MC4-R + PRV colocalization (∼80%), and IBAT temperature was measured via temperature transponders implanted below this tissue as a direct assessment of BAT thermogenesis in a separate set of animals. In principle, therefore, parenchymal MTII microinjections into the PVH should stimulate the MC4-Rs expressed by PRV infected SNS outflow neurons to IBAT and thereby increase IBAT temperature, although we cannot be absolutely certain the same cells previously labeled by PRV in other animals were stimulated in this additional functional study. Note that while the present work was in preparation, it was demonstrated that PRV injected into IBAT of genetically engineered MC4R-green fluorescent protein (GFP) mice infected MC4-R-GFP cells in similar brain areas to those reported here (56); however, additional brain sites of sympathetic outflow neurons possessing MC4-R mRNA were found in the present experiment.
METHODS
Experiment 1: Colocalization of MC4-R mRNA with PRV-labeled SNS Outflow Neurons to IBAT
Animals.
Procedures for experiments 1 and 2 were approved by the Georgia State University Institutional Animal Care and Use Committee and are in accordance with Public Health Service and the U.S. Department of Agriculture guidelines. In experiment 1, adult male Siberian hamsters ∼3 mo old (n = 5) were obtained from our breeding colony, the lineage of which has been described recently (10). Hamsters were group housed and exposed to a long-day photoperiod (16:8-h light-dark cycle; lights on at 0200) from birth. Room temperature was maintained at 21 ± 2°C. They were fed PMI Rodent Diet no. 5001 (Purina, St. Louis, MO) throughout the experiment and were single-housed 1 wk before undergoing any procedure.
PRV injections.
Animals were anesthetized with pentobarbital sodium (50 mg/kg), and the target incision area was shaved and wiped with 50% ethanol. A small incision was made to expose both IBAT pads. Care was taken with the depth of the incision so as not to damage the underlying fat pad and vasculature. The application of the PRV was performed essentially as described previously (4). Briefly, once the left IBAT pad was exposed, a series of injections of PRV 152 (PRV strain with GFP; generous gift of Lynn Enquist, Princeton, NJ) was made using a 1.0-μl microsyringe at five loci within the left IBAT (7.5 × 107 pfu/ml; 150 nl/loci) to evenly distribute the virus. The incision was closed with sterile wound clips and nitrofurozone powder (nfz Puffer; Hess & Clark, Lexington, KY) was applied to minimize the risk of infection. The animals were then transferred to clean cages and were maintained for 6 days before death, the optimal postinoculation survival time for infection to reach the rostral forebrain from this fat pad (4).
General histology.
Six days postinjection, the animals were given an overdose of pentobarbital sodium (300 mg/kg) and perfused transcardially with heparinized (0.02%) saline and phosphate-buffered (0.1 M; pH 7.4) paraformaldehyde (4% wt/vol). The brains and spinal cords were extracted, postfixed in the same fixative overnight at 4°C, and sunk in sucrose (30% wt/vol; with 0.1% sodium azide) the next day. The brains were sliced at 25 μm on a cryostat and the sections immersed in cryoprotectant at −20°C until being further processed. The spinal cords were sliced on a freezing stage sliding microtome at 50 μm in the horizontal plane. The spinal cord sections were then mounted onto gelatin-coated slides and coverslipped with Vectashield (Vector Laboratories, Burlingame, CA). The thoracic and lumbar spinal cord regions were scanned for the presence of lysed cells or bilateral infection, both signs of nonspecific PRV infections, by examining the sections for the PRV152-induced GFP reporter using an Olympus BX41 microscope with a green fluorochrome filter.
Combined in situ hybridization/immunohistochemistry.
The protocol for combined in situ hybridization and immunohistochemistry for MC4-R mRNA + PRV-ir used in this study is identical to one detailed previously for MC4-R mRNA + PRV-ir in WAT (53). Every third brain section was processed for combined in situ hybridization and immunohistochemistry for MC4-R mRNA and PRV-immunoreactivity (ir), respectively.
Probe preparation.
Plasmids containing the 289-bp cDNA sequence corresponding to nucleotides 707–995 of the published sequence of rat MC4-R (GenBank accession no. U 67863.1) were linearized with HindIII and XhoI restriction enzymes (Promega, Madison, WI) and transcribed in vitro using T7 and SP6 RNA polymerases (Stratagene, La Jolla, CA) in the presence of 35S-labeled UTP (PerkinElmer, Boston, MA) to generate sense and antisense probes, respectively. The specifics of this sequence including the cloning procedure followed are detailed in our previous report (53), where we describe colocalization of MC4-R mRNA with PRV-infected SNS outflow neurons when PRV is injected into inguinal WAT.
In situ hybridization for MC4-r.
Brain sections were rinsed in 2× SSC (1× = 0.15 M sodium chloride and 0.015 M sodium citrate), dehydrated in ascending concentrations of ethanol, delipidated with chloroform, rehydrated in descending concentrations of ethanol, deaminated in acetic anhydride (0.25%)-triethanolamine (0.1 M in saline), and then incubated in formamide (50%; with 2× SSC, 0.1% sodium dodecyl sulfate, 25 mM dithiothreitol). The sections were then hybridized with 35S-labeled MC4-R cRNA or sense control probes (30,000 cpm/μl) at 57°C overnight. The following day, the sections were cooled to room temperature, rinsed in 1× SSC, 2× SSC-formamide (1:1, 30 min at 53°C), incubated in RNase A (100 mg/ml), washed in 2× SSC-formamide (1:1, 90 min at 57°C), and rinsed in 1× SSC and in PBS (0.1 M; pH 7.4) for immunohistochemical processing to detect PRV in the same sections.
Immunohistochemistry for PRV.
Sections were incubated sequentially in the primary antibody (Rb132, 1:10,000; a generous gift of Lynn Enquist, Princeton, NJ) overnight, secondary antibody (goat anti-rabbit; 1:500; Vector Laboratories) for 2 h and in avidin-biotin complex (1.75 μl/ml each of avidin and biotin) for 1 h. The specific labels were detected using diaminobenzidine (0.1 mg/ml; Sigma, St. Louis, MO) as the chromogen in the presence of hydrogen peroxide (0.0025%). All steps in the immunohistochemistry procedure were performed at 22°C. The sections were mounted onto gelatin-coated slides, rinsed in deionized water and ethanol, and then air dried. Isotopic labels were detected autoradiographically by dipping sections into NTB3 liquid emulsion (Eastman Kodak, Rochester, NY). Slides were exposed for 5 days at 4°C, developed and fixed in D-19 and Fixer (Eastman Kodak), respectively, counterstained with Cresyl Violet, and coverslipped.
Data analysis and imaging.
All brain sections processed were analyzed (Olympus BX41 microscope) for single-labeled PRV and double-labeled [MC4-R + PRV] cells in all nuclei where PRV infection was found from the level of the brain stem approximating Plate 100 in the mouse brain atlas (40) at the caudal extent through the medial preoptic area [POA; Plate 26 (40)]. PRV-infected neurons were considered double labeled if the autoradiographic granular deposition above the cells was at least seven times that of background levels in adjacent areas within the same sections and conformed to the shape of the cells. Images were captured digitally with an Olympus DP70 camera and acquired using Photoshop (version 6.0; Adobe, San Jose, CA). A mouse brain atlas (40) was used to identify brain regions, as no brain atlas exists for Siberian hamsters. PRV-infected neurons and the subpopulation of PRV cells that also were labeled for MC4-R mRNA ([MC4-R + PR]) were counted. Data were collapsed across each bilateral nucleus/region within each animal and then averaged across the animals.
Experiment 2: Stimulation of Melanocortin Receptors with MTII to Activate IBAT Thermogenesis
Animals.
Adult male Siberian hamsters (n = 23; ∼3 mo old) were obtained from our breeding colony, with housing and care as described in experiment 1, except where noted.
Surgery.
Cannula and transponder implantation were done in a single surgery. Hamsters were anesthetized with isoflurane (Baxter Healthcare, Deerfield, IL), and fur on the head and the dorsal surface of the body was removed before surgery to expose areas of interest. A midline incision along the upper dorsal surface of the body was made and the IBAT pads were exposed. A temperature transponder [Implantable Programmable Temperature Transponder (IPTT)-300; BioMedic Data Systems, Seaford, DE] was tied to sterile silk sutures and then affixed to the left IBAT pad with the temperature-sensitive portion of the IPTT-300 directed at the medial border of the left IBAT pad. The IBAT pad was moistened with sterile saline, then the incision was closed with sterile wound clips, and nitrofurozone was applied to minimize the risk of infection.
A guide cannula (26-gauge stainless steel; Plastics One, Roanoke, VA) was lowered for stereotaxic placement just above the left PVH (coordinates: 0.03 mm posterior to bregma, 0.03 mm to the left of the midsaggital sinus, and −5.5 mm from the top of the level skull). The guide cannula and two Jeweler's screws (3/16 mm) placed ∼5 mm apart were secured to the skull with cyanoacrylate ester gel glue and dental acrylic (Ortho-Jet; Lang Dental, Wheeling, IL). A removable obturator (Plastics One, Roanoke, VA) sealed the opening in the guide cannula throughout the experiment, except during injections. Hamsters received buprenorphine (0.2 mg/kg; Reckitt Benckiser Pharmaceuticals, Richmond, VA) upon arousal from anesthesia to minimize postoperation discomfort. They recovered for ∼1 wk before testing.
To determine a dose range of MTII to inject parenchymally, we used doses that were ineffective when injected into the third ventricle so as to control for potential leakage from the site-specific site into the ventricular system. We previously have shown that 5 nmol MTII maximally stimulated IBAT temperature, with a smaller, but significant increase in IBAT temperature at 0.5 nmol (11); therefore, we began testing with doses lower than this dose. Thus, in a pilot study, 12 hamsters of the same age and body mass range of the present study had guide cannulae stereotaxically implanted into the third ventricle and, after a 7-day recovery, received an injection of 0 (saline vehicle) or 0.05, 0.10, 0.20, or 0.30 nmol MTII (Phoenix Pharmaceuticals, Burlingame, CA) in a cross-over design where each hamster got at least one of the MTII doses or saline. The injection protocol and transponder implantation (see methods) and verification (see methods) were identical to the description for the formal experiment. None of these doses were in a range that consistently or persistently significantly increased IBAT temperature (Table 1). Therefore, we chose MTII doses for parenchymal microinjections that were in the lower portion of this range to insure that even if the entire volume leaked into the third ventricle, IBAT temperature would likely not increase (i.e., 0.025, 0.05, and 0.075 nmol).
Table 1.
Results of a pilot study to determine third ventricular injections of melanotan II (MTII) that do not increase interscapular brown adipose tissue (IBAT) temperature
| Dose | 1 h Postinjection | 2 h Postinjection | 3 h Postinjection | 4 h Postinjection | No. |
|---|---|---|---|---|---|
| Saline | 36.8±0.2 | 37.3±0.2 | 37.3±0.2 | 37.1±0.2 | 12 |
| MTII, 0.05 nmol | 37.0±0.6 | 37.1±0.8 | 37.0±0.3 | 36.9±0.4 | 4 |
| MTII, 0.10 nmol | 37.2±0.2 | 37.3±0.1 | 37.2±0.2 | 36.8±0.2 | 6 |
| MTII, 0.20 nmol | 37.2±0.2 | 37.3±0.5 | 37.5±0.4 | 37.2±0.1 | 3 |
| MTII, 0.30 nmol | 37.8±0.3 | 37.7±0.3 | 37.0±0.5 | 36.8±0.3 | 3 |
Note that we previously reported that third ventricular injections of 5 nmol MTII maximally stimulated IBAT temperature, with a smaller, but significant increase in IBAT temperature at 0.5 nmol (11); therefore, we began testing with doses lower than this latter dose.
Experimental design.
Following recovery, hamsters were divided into control and experimental groups matched on their body mass and percent change in body mass from single housing. They were handled for three consecutive days before the experimental procedure to acclimate them to handling during injections. All hamsters received all treatments according to a Latin square design with 1 day between test days. On test days, the portable hand-held reader-programmer (DAS-5002 Notebook System; BioMedic Data Systems, Seaford, DE) was used to scan the IBAT temperature of each hamster by passing a wand above the animal's dorsum (i.e., above the IBAT pad with the affixed transponder) in its home cage.
Test day injection protocol.
Food from the home cage and cheek pouches, as well as nestlets, was removed at 0600, with water remaining available. At 0800, hamsters were injected with 200 nl of MTII or saline vehicle through the implanted guide cannula using an inner cannula (33-gauge stainless steel; Plastics One) that was custom cut to extend 0.5 mm beyond the tip of the guide cannula and connected at the other end to a microsyringe via polyethylene tubing that was backfilled with deionized water. Animals were lightly restrained by hand for 30 s during injection, and the injection needle was kept in place for an additional ∼30 s before removal to minimize backward efflux. On test days, two IBAT temperature scans were made during the baseline adaptation period (0600 and 0700), one scan made just before the parenchymal injection (0800) and then once hourly for the next 4 h (0900, 1000, 1100, and 1200). Food and fresh nestlets were returned after the last temperature reading of each test day.
Transponder and cannula verifications.
One day following the last drug test, hamsters were injected with 0.8 mg/kg norepinephrine (Sigma Aldrich, St. Louis, MO) subcutaneously to verify transponder function. At the end of the experiment, hamsters were overdosed with pentobarbital sodium (300 mg/kg) and the transponder location was verified visually by examining the position of the copper portion of the transponder relative to the medial border of the left IBAT pad. Cannula placement was verified by injecting 200 nl of toluidine blue dye into the PVH. Following transponder verification and injection of dye, hamsters were perfused transcardially with heparinized (0.02%) saline and phosphate buffered (0.1 M; pH 7.4) paraformaldehyde (4% wt/vol). Brains were extracted and sliced using a freezing stage sliding microtome at 80 μm. Cannulae placement were determined by superimposing digital images of brain slices containing the dye-stained cannulae tracts over corresponding plate images from the mouse brain atlas (40) using Adobe Photoshop (version 7.0). All hamsters with functional, correctly placed transponders and confirmed PVH cannulae placements were included in the analyses.
Statistics.
Absolute temperature data were analyzed by a two-way repeated-measures ANOVA (dose × time = 4 × 4) with post hoc comparisons made when appropriate using Tukey-Kramer multiple comparison tests (NCSS, Kaysville, UT). Differences among group means were considered statistically significant if P < 0.05. Exact probabilities and test values are not included for simplicity and clarity of the presentation of the data.
RESULTS
Experiment 1: Colocalization of MC4-R mRNA with PRV-labeled SNS Outflow Neurons to IBAT
PRV infection.
There were no overt signs of sickness in the animals other than diminished nesting and grooming behaviors just before termination on day 6. There also were no cases of nonspecific infections (lysed cells or bilateral spinal cord infection). PRV 152 injected into IBAT resulted in PRV infection pattern across the neuroaxis, similar to what we reported previously when Bartha's PRV (parental strain) was used (4). There was unilateral infection of the lumbar and thoracic spinal cord, including the intermediolateral horn, ipsilateral to the injected IBAT pad. In the brain stem, PRV-labeled cells were found most notably in brain stem raphé regions [e.g., dorsal, raphé pallidus (RPa), raphé obscurus (ROb)], gigantocellular and lateral paragigantocellular (LPGi) areas, reticular regions (including the lateral and interstitial subdivisions), facial nerve and trigeminal areas and the Sol. Midbrain infection was highly consistent along the rostrocaudal extent of the periaqueductal gray (PAG) and to a lesser extent in the pedunculopontine tegmental, pontine, and deep mesencephalic nucleus, as well as in the ventral tegmental area. Forebrain PRV infection was most impressive in the PVH, primarily in the medial magnocellular and posterior part subdivisions, POA, lateral hypothalamus, including the region below the zona incerta (subzona incerta; SubZi), posterior hypothalamus, dorsomedial hypothalamus and, to a lesser extent, in the Arc and on the fringes of the ventromedial hypothalamus. There also was scant labeling in the suprachiasmatic nucleus (SCH). Thalamic infections were predominantly in the reuniens, particularly in the ventral reuniens.
PRV infections in MC4-r expressing cells.
As expected, sense control probes caused no specific autoradiographic labeling (data not shown). The distribution of MC4-R mRNA labeling found in this study was similar to what we reported previously in Siberian hamsters for PRV injected into WAT (53) and confirms and extends the general pattern of distribution reported in laboratory rats (31) and in mice (34) for PRV injected into BAT. Furthermore, there was extensive colocalization of PRV-ir and MC4-R mRNA (MC4-R + PRV) in cells across the neuroaxis. The data are expressed as the percentage of the PRV-ir cells in these nuclei/regions exhibiting MC4-R mRNA {([MC4-R + PRV] ÷ PRV)·100; Table 2 }. The ratio of colocalized cells ranged from as low as ∼38% in the periventricular fiber system to as high as ∼98% in the paramedian raphé nucleus (PMnR; Table 2). The number of PRV-infected cells was not predictive of the level of colocalization. For example, the highest and lowest colocalization ratios found were in areas that had very few infected cells (paramedian raphé: ∼98% at ∼12 total infected cells, periventricular fiber system: ∼38% with ∼21 total infected cells), whereas areas with very high infections (>1,000 cells), such as the PVH and brain stem PAG areas had ∼84 and ∼82% [MC4-R + PRV] cells, respectively (Table 2).
Table 2.
Distribution of PRV and PRV + MC4-R mRNA after IBAT injection of PRV
| PRV | [PRV + MC4-R]÷PRV% | PRV | [PRV + MC4-R]÷PRV% | ||||
|---|---|---|---|---|---|---|---|
| Forebrain | VLPAG | 599.4±200.67 | 82.64±0.6 | ||||
| Pontine | 464.20±209.81 | 78.48±2.15 | |||||
| Hypothalamic | PPTg | 322.20±146.74 | 85.79±3.82 | ||||
| AH/LA | 102.80±38.14 | 76.08±3.29 | pv | 20.75±10.86 | 38.03±19.94 | ||
| Arc | 108.20±44.68 | 77.86±6.15 | R | 64.00±32.87 | 83.47±2.66 | ||
| DM | 181.20±73.88 | 79.78±5.07 | RRF/A8/rs | 26.75±14.14 | 59.97±19.13 | ||
| DTM | 17.75±4.84 | 90.03±3.25 | RtTg | 12.50±2.21 | 90.63±5.93 | ||
| LH | SN | 12.20±5.62 | 87.39±6.52 | ||||
| SUBZi | 490.00±161.31 | 80.46±1.28 | SPTg | 55.00±17.06 | 81.16±3.28 | ||
| Pe/AVPe | 417.40±113.54 | 82.84±1.97 | VLTg | 27.33±4.84 | 81.33±8.46 | ||
| PH | 219.60±87.70 | 85.74±4.53 | VTA | 201.00±55.48 | 88.76±2.10 | ||
| POA | 589.40±230.30 | 76.79±1.54 | Xscp | 12.75±9.68 | 84.52±6.05 | ||
| PVN | 1172.60±308.83 | 84.22±2.02 | Brainstem | ||||
| PaAP | 48.20±20.80 | 79.70±3.78 | |||||
| PaDC | 20.50±6.64 | 85.16±3.26 | 12 | 9.67±2.70 | 79.63±3.59 | ||
| PaLM | 183.60±50.95 | 83.41±3.37 | 10 (DMV) | 22.50±0.95 | 86.90±2.26 | ||
| PAMM | 374.20±122.87 | 83.23±2.91 | A5 | 285.40±91.51 | 79.47±1.52 | ||
| PaMP | 204.20±86.87 | 82.65±2.65 | Amb | 135.75±52.00 | 81.07±2.12 | ||
| PaPo | 366.25±148.94 | 85.59±3.95 | AP | 71.40±21.19 | 72.14±2.21 | ||
| PaV | 61.20±18.09 | 80.27±5.05 | Bar | 50.75±25.97 | 81.60±2.39 | ||
| RCh | 14.33±7.28 | 73.30±3.47 | Facial | 280.60±91.42 | 76.72±2.49 | ||
| Spa | 10.67±3.80 | 74.32±10.42 | DPGi | 117.00±21.19 | 80.12±2.87 | ||
| SCH | 30.67±6.93 | 68.51±1.49 | LPGi | 678.20±166.75 | 84.34±1.92 | ||
| VMH | 74.00±23.38 | 71.52±1.76 | Gi | 482.00±153.16 | 86.90±4.35 | ||
| Thalamic | GiA | 274.80±72.38 | 83.05±2.27 | ||||
| GiV | 316.20±68.24 | 91.98±1.16 | |||||
| LHb | 14.50±3.48 | 81.94±4.39 | KF | 53.75±8.66 | 82.66±5.24 | ||
| PrC | 58.00±25.97 | 77.97±1.11 | LC | 168.40±60.17 | 80.67±2.31 | ||
| PSTh | 23.50±5.38 | 80.63±0.40 | Li | 84.33±8.05 | 90.54±0.49 | ||
| PV | 18.25±5.14 | 93.84±3.95 | LPB | 100.75±31.63 | 75.30±7.74 | ||
| Re/Vre/Xi | 139.75±55.63 | 81.71±2.53 | MPB | 82.20±24.60 | 82.99±5.24 | ||
| Rt | 12.00±3.66 | 86.83±2.65 | PBP | 49.00±15.81 | 82.71±7.51 | ||
| Other Forebrain | Ppy | 53.25±16.81 | 76.32±2.79 | ||||
| Pr | 196.40±45.77 | 78.78±5.72 | |||||
| AC | 11.00±2.93 | 74.07±6.75 | |||||
| Amygdala | 14.50±1.58 | 88.24±7.44 | Raphé | ||||
| BST | 114.00±56.58 | 83.70±2.16 | DR | 248.60±94.86 | 79.45±5.50 | ||
| HDB/VDB | 18.50±6.64 | 70.91±5.32 | MnR | 13.50±2.85 | 77.78±0.00 | ||
| Fascicular | 26.40±9.91 | 50.49±13.42 | PMR | 12.40±10.43 | 97.78±2.22 | ||
| LS | 5.00±1.32 | 93.30±3.47 | Rli/Cli | 23.60±9.36 | 78.03±5.25 | ||
| PeF | 17.50±2.21 | 85.71±9.04 | RC | 208.80±65.15 | 82.97±2.79 | ||
| Mammillary bodies | 20.80±16.67 | 85.47±9.19 | RMg | 224.40±67.06 | 76.33±2.93 | ||
| SI | 14.50±4.74 | 86.36±8.62 | Rob | 343.00±85.00 | 68.57±17.16 | ||
| TC | 38.00±8.93 | 69.73±6.89 | Rpa | 605.80±97.51 | 85.16±2.32 | ||
| ZI | 60.50±24.87 | 87.03±5.83 | Reticular | ||||
| Midbrain | Md-D/V | 165.20±70.57 | 85.06±3.85 | ||||
| CnF | 98.20±70.48 | 81.65±6.20 | Irt | 292.60±120.97 | 81.22±1.96 | ||
| Dk | 19.50±4.74 | 96.30±2.34 | LRt | 376.80±99.86 | 82.25±1.85 | ||
| DMTg | 53.25±20.92 | 78.02±2.97 | PCRt | 42.33±18.62 | 86.39±5.27 | ||
| DpMe | 267.60±88.84 | 87.53±3.42 | PCRtA | 67.40±18.70 | 82.11±5.13 | ||
| DTg | 18.50±11.07 | 97.22±1.76 | PMn | 111.80±60.93 | 86.43±4.19 | ||
| EW | 5.00±0.37 | 76.67±7.89 | RVL | 229.40±80.62 | 76.97±3.79 | ||
| F | 1.00±0.00 | 50.00±31.62 | Sol | 532.80±152.44 | 82.40±4.60 | ||
| LDTg/-V | 188.40±41.63 | 85.14±2.78 | Sub-cd/v | 123.80±49.50 | 81.40±5.47 | ||
| Lemniscus | 77.00±46.18 | 79.46±5.36 | Trigeminal | 295.40±70.91 | 80.80±1.52 | ||
| Oculomotor | 52.80±25.47 | 89.82±3.83 | Vestibular | 90.40±38.88 | 77.51±2.98 | ||
| Olivary | 95.60±49.91 | 82.85±5.79 | Vsc | 1027.20±293.29 | 73.89±3.62 | ||
| Pa4 | 15.00±8.22 | 94.64±3.39 | |||||
| Pc/Pcom | 30.80±14.80 | 87.18±4.99 | |||||
| PAG | 1337.60±491.95 | 82.51±0.87 | |||||
| DLPAG | 85.5±38.42 | 78.39±4.26 | |||||
| DMPAG | 229.6±87.90 | 85.16±4.07 | |||||
| LPAG | 308.2±131.73 | 85.90±3.62 | |||||
Values are means ± SE. PRV, pseudorabies virus; MC4-R, melanocortin 4 receptors; AH, anterior hypothalamus; Arc, arcuate nucleus; DM, dorsomedial nucleus; DTM, dorsal tuberomammillary; LH, lateral hypothalamus; Pe/AVPe, periventricular/anteroperiventricular; PH, posterior hypothalamus; POA, preoptic area; PVN, paraventricular hypothalamus; PaAP, paraventricular hypothalamic anterior parvicellular; PaDC, parventricular hypothalamic dorsal cap; PaLM, parventricular hypothalamic lateral magnocellular; PaMM, paraventricular hypothalamic medial magnocellular; PaMP, paraventricular hypothalamic medial parvocellular; PaPo, paraventricular hypothalamic posterior; PaV, paraventricular hypothalamic ventral part; RCh, retrochiasmatic area; Spa, subparaventricular nucleus; SCH, suprachiasmatic nucleus; SubZi, sub-zona incerta of the LH; VMH, ventromedial hypothalamus; LHb, lateral habenular; PrC, precommissural nucleus; PSTH, parasubthalamic nucleus; PV, parventricular thalamus; Re/Vre/Xi, reuniens/reuniens ventral/xiphoid; Rt, reticular thalamic nucleus; AC, anterior commissural nucleus; BST, bed nucleus of the stria terminalis; HDB/VDB, diagonal band-horizontal limb/vertical limb; LS, lateral septum; ns, nigrostriatal bundle; PeF, perifornical nucleus; SI, substantia innominata; TC, tuber cinerium; ZI, zona incerta; A8, dopaminergic cell field; CnF, cuneiform; Dk, darkschewitsch; DMTg, dorsomedial tegmental area; DpMe, deep mesencephalic nucleus; DTg, dorsal tegmental; EW, Edinger Westphal; F, nucleus of the fields of Forel; LDTg/-V, laterodorsal tegmental/-ventral part; Pa4, paratrochlear; Pc/Pcom, postcommissural/nucleus; PAG, periaqueductal gray; PPTg, pedunculopontine tegmental nuclei; pv, periventricular fiber system; R, red nucleus; RRF, retrorubral field; rs, rubrospinal tract; RtTg, reticulo temental pons; SN, substantia nigra; SPTg, subpeduncular tegmental nucleus; VLTg, ventrolateral tegmental nucleus; VTA, ventral tegmental area; xscp, decussation of the superior cerebellar peduncle; 10 DMV, dorsal motor nucleus of the vagus nerve; 12, hypoglossal nucleus; A5, noradrenergic cell field; Amb, ambiguous nucleus; AP, area postrema; Bar, Barrington's nucleus; DPGi, dorsal paragigantocellular nucleus; Gi, gigantocellular reticular nucleus; GiA, gigantocellular reticular anterior; GiV, gigantocellular reticular ventral; KF, Kolliker fuse; LC, locus coeruleus; Li, linear nucleus of the medulla; LPB, lateral parabrachial nucleus; LPGi, lateral paragigantocellular nucleus; MPB, medial parabrachial nucleus; PBP, parabrachial pigmented; Ppy, peripyramidal nucleus; Pr, prepositus hypoglossal nucleus; DR, dorsal raphé; MnR, median raphé; PMnR, paramedian raphé; RC, raphé cap; Rli/Cli, rostral linear nucleus of the raphé/caudal linear nucleus of the raphé; RMg, raphé magnus nucleus; ROb, raphé obscurus; RPa, raphé pallidus; Irt, intermediate reticular nucleus; LRt, lateral reticular nucleus; MdD-/V, medullary nucleus dorsal-/ventral; PCRt-/A, parvicellular reticular nucleus/anterior; PMn, paramedian reticular nucleus; RVL, rostroventrolateral reticular nucleus; Sol, solitary tract nucleus; SubCD/-V, subcoeruleus nucleus; dorsal/-ventral; vsc, ventral spinocerebellar tract.
In the forebrain, high levels of colocalization were found in numerous regions including the POA (∼77%; Fig. 1), PVH (∼84%; Fig. 2, A and B), subparaventricular zone (Spa; ∼74%), dorsomedial hypothalamus (∼80%), posterior hypothalamus (∼86%; Table 2). There also were MC4-R mRNA + PRV cells in the Arc (∼78%), ventromedial hypothalamus (∼72%), and the SCH (∼69%), although to a much lower extent in terms of the total number of infected cells in these areas (e.g., ∼31 cells in the SCH to ∼108 cells in the Arc; Table 2). In the thalamus, the highest percentage of colocalization was in the paraventricular thalamus (∼94%), but this area had a low absolute number of double-labeled cells (∼18; Table 2). By contrast, the thalamic area with the highest level of infection was the reuniens/ventral reuniens/xiphoid region (∼140 cells) with a lower percentage of colocalization than for the paraventricular thalamus (∼82%; Table 2).
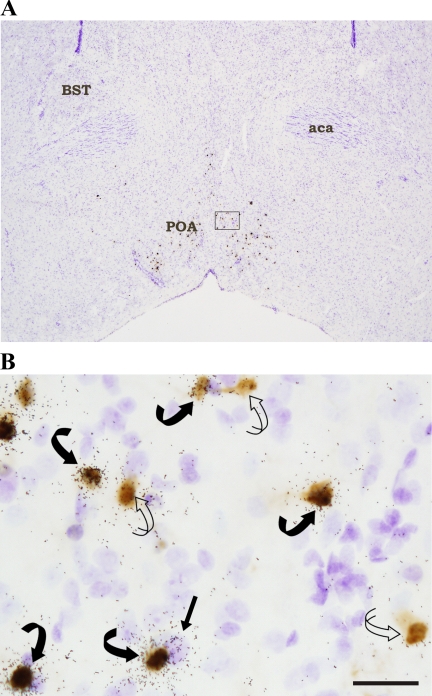
Fig. 1.
A: low magnification photomicrograph of a hypothalamic section from a hamster injected with pseudorabies virus (PRV-152) into interscapular brown adipose tissue (IBAT). This section approximating Plate 27 of the mouse brain atlas (40) was processed for combined immunocytochemistry and in situ hybridization to reveal PRV and melanocortin 4 receptor (MC4-R) mRNA labeling, respectively, and was then counterstained (Cresyl Violet; blue). The outlined area of the preoptic area (POA) is enlarged in B. B: PRV-152-infected cells [diaminobenzidine (DAB); brown] can be seen along with MC4-mRNA-positive (autoradiography; black granules) cells in the POA. There also were PRV-infected cells double labeled for MC4-R mRNA (i.e., [MC4-R + PRV]). Curved open arrow = PRV (brown; DAB); straight black arrow = MC4-R mRNA (black autoradiography granules over blue from Cresyl Violet counterstain); curved black arrows = [MC4-R + PRV] cells (black granules over brown DAB). Arrows indicate representative cells within the section only. aca, Accumbens, anterior; BST, bed nucleus, stria terminalis. Bar = 25 μM.
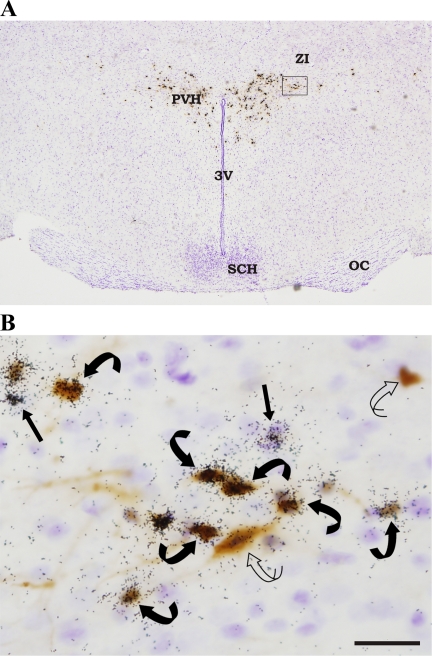
Fig. 2.
A: low magnification photomicrograph of a hypothalamic section (approximating Plate 38–39, Ref. 40) stained for PRV and for melanocortin 4-receptor (MC4-R) mRNA and then counterstained with Cresyl Violet. B: portion of the paraventricular nucleus of the hypothalamus (PVH) outlined in Fig. 2A is enlarged. Curved open arrow = PRV (brown); straight black arrow = MC4-R mRNA (autoradiography black granules); curved black arrows = [MC4-R + PRV] cells (black granules over brown DAB). Arrows indicate representative cells within the section. ZI, zona incerta; SCH, suprachiasmatic nucleus; 3V, third ventricle; OC, optic chiasm. Bar = 25 μM.
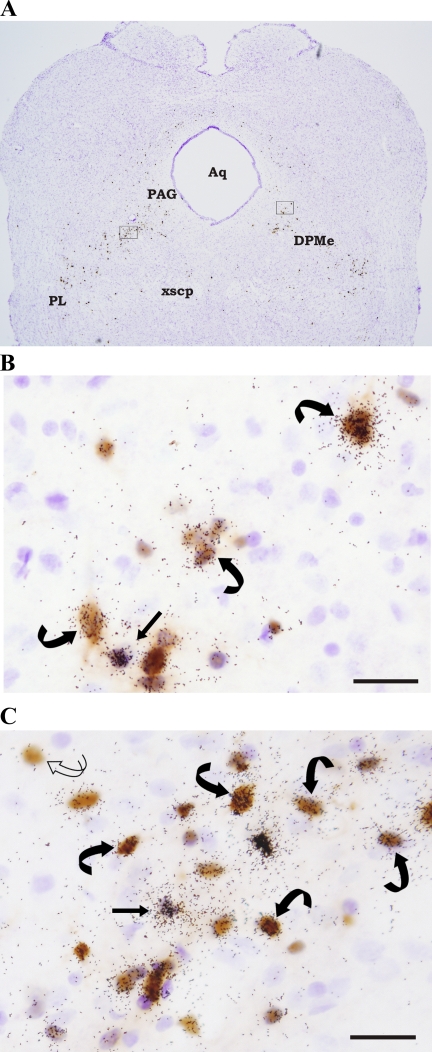
Although there was much less PRV infection in the midbrain (Fig. 3A), in general, many of these areas had colocalization ratios between ∼80 and 90% (Table 2). For example, in the PAG (Fig. 3B), ∼82% of the substantial ∼1,338 PRV infected cells also were labeled for MC4-R mRNA (Table 2). In the deep mesencephalic nucleus, despite a much more conservative number of total PRV-infected cells (267.6 ± 88.4), ∼87% of these cells were double labeled for PRV and MC4-R mRNA (Fig. 3C; Table 2).
Fig. 3.
A: low magnification photomicrograph of a hamster midbrain section (approximating Plate 69, Ref. 40) double labeled for PRV and MC4-R mRNA. A majority of the midbrain PRV and [MC4-R + PRV] cells were found within the periaqueductal gray (PAG) and deep mesencephalic nucleus (DPMe). Outlined portions of the PAG and DPMe are enlarged in B and C. B: [MC4-R + PRV] cells in the PAG. A single-labeled MC4-R mRNA cell also is shown. C: PRV and MC4-R mRNA labeling in the DPMe. Single-labeled PRV (curved open arrow), single-labeled MC4-R mRNA (straight black arrow), and double-labeled [MC4-R + PRV] (curved black arrows) cells also can be seen in the midbrain DPMe. Arrows indicate representative cells. Aq: aqueduct; PL: paralemniscal nucleus; xscp: decussation of the superior cerebellar peduncle. Bar = 25 μM.
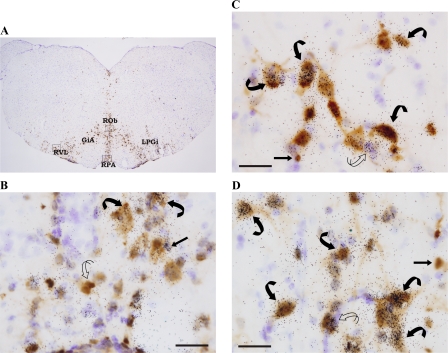
In the brain stem, several areas had high percentages of numbers of PRV infected cells colabeled with MC4-R mRNA (Fig. 4, Fig. 5), such as the RPA (∼85%; Fig. 4B), rostroventrolateral medulla (∼77%; Fig. 4C), LPGi (84%), ROb (∼70% Fig. 4D), and the Sol (∼82% of ∼500 cells; Fig. 5, A and B; Table 2). There also were [MC4-R + PRV] cells found in the dorsal motor nucleus of the vagus nerve (∼87% of ∼20 cells; Fig. 5C, 10), almost exclusively located in its outermost edges, adjoining the Sol (Table 2).
Fig. 4.
A: photomicrograph of hamster brain stem section processed for PRV and MC4-R mRNA (approximating Plate 85, Ref. 40). Areas outlined in Fig. 4A are enlarged for cellular resolution of the single- and double-labeled cells in the raphé pallidus (RPA; B), rostroventrolateral reticular nucleus (RVL; C), and raphé obscurus (ROb; B). Single-labeled PRV (curved open arrow), single-labeled MC4-R mRNA (straight black arrow), and double-labeled [MC4-R + PRV] (curved black arrows) cells can also be seen. Arrows indicate representative cells. GiA, gigantocellular reticular nucleus, alpha; LPGi, lateral paragigantocellular nucleus. Bar = 25 μM.
Fig. 5.
A: photomicrograph of hamster brain stem approximating Plate 94 (40). B: PRV infection and [MC4-R + PRV] cells are abundant in the nucleus of the solitary tract (Sol). By contrast, the comparatively low levels of PRV and [MC4-R + PRV] labeling in the dorsal motor nucleus of the vagus nerve (DMV, 10) is shown in Fig. 5C. C: PRV infection in the DMV is predominantly in the outer edges adjoining the Sol (as shown here) and may be rogue neurons of the sympathetic nervous system (see Ref. 22). Single-labeled PRV (curved open arrow), single-labeled MC4-R mRNA (straight black arrow), and double-labeled [MC4-R + PRV] (curved black arrows) cells also can be seen. Arrows indicate representative cells. AP: area postrema; Irt: intermediate reticular nucleus; LRt: lateral reticular nucleus. Bar = 25 μM.
Experiment 2: Stimulation of Melanocortin Receptors with MTII to Activate IBAT Thermogenesis
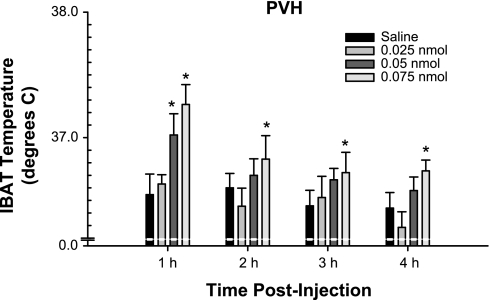
No animals were eliminated because of misplaced transponders or due to a lack of norepinephrine-induced increased IBAT temperature (increases of at least 1.5°C). MTII injected into the PVH at 0.05 and 0.075 nmol significantly increased IBAT temperature 1 h postinjection compared with saline (P < 0.05; Fig. 6). Moreover, the highest dose of MTII (0.075 nmol) elevated IBAT temperature at 2, 3, and 4 h postinjection (Fig. 6; P < 0.05).
Fig. 6.
Mean ± SE IBAT temperature (°C) after PVH injection of melanotan II (MTII) or saline. *P < 0.05 vs. saline.
DISCUSSION
The results of the present experiment demonstrate extensive colocalization of MC4-R mRNA with cells that are part of the sympathetic outflow from brain to IBAT across the neuroaxis in Siberian hamsters, as double labeled by in situ hybridization and PRV-ir, respectively. These data extend and expand similar findings reported while this work was in progress, showing high levels of colocalization of PRV-infected neurons in the brain after injection into IBAT of MC4-R-GFP transgenic mice (56). Moreover, here we show that microinjection of the MC3/4-R agonist, MTII into the PVH, an area implicated in energy expenditure (e.g., 9, 20, 60) and showing high colocalization of MC4-R + PRV cells here (∼80%), significantly increased IBAT temperature.
The findings by Voss-Andreae et al. (56), where PRV was injected into IBAT of MC4-R-GFP transgenic mice, are highly consistent with the findings of the present study. The degree of colocalization found in that study, however, is modest compared with the findings in the present study. For example, the two highest percentages of PRV infected cells that coexpress MC4-R in the MC4-R-GFP transgenic mice after the longest post-PRV-injection time (i.e., 5 days) was in the laterodorsal tegmental nucleus and the RPa with 73% and 42% colocalization, respectively (56), and, in our case for these areas, it was ∼85% for both (Table 2). Perhaps this and many other brain sites showing greater degrees of colocalization in the present study vs. Voss-Andreae et al. can be explained by the different species used (mice vs. Siberian hamsters), different histological approaches (MC4-R-GFP transgenic mice + PRV vs. in situ hybridization for MC4-R + PRV-ir), and different PRV strains and viral loads used (2 × 109 pfu of PRV-Bablu vs. 7.5 × 107 pfu/ml PRV 152). Despite these differences in our approaches and quantitative results, however, our findings in the present study nonetheless confirmed the brain sites identified in the transgenic mouse study and further expanded the list of sites that are part of the sympathetic outflow to IBAT that also express MC4-R mRNA.
The degree of colocalization of MC4-R mRNA with sympathetic outflow neurons to IBAT reported here is reminiscent of the similarly high levels of colocalization of MC4-mRNA with sympathetic outflow neurons to WAT we found previously (53). That is, in the present work, the degree of colocalization was often ≥ 70% and most often between 80 and 90% (Table 2), whereas in the complementary study of WAT sympathetic neurons expressing MC4-R mRNA, the degree of colocalization was somewhat less at typically ∼60% or greater with many sites in the 70–80+% range (53). Such high degrees of colocalization are rare in studies employing viral tract tracers to label circuits to any tissue (for a review, see Ref. 48), most likely because viral tracers, such as PRV, hijack the host cellular machinery to propagate their virion progeny, thereby resulting in a suppression of the synthesis of many substances normal to the host cell (e.g., neurotransmitters, receptors; for a review, see Ref. 52). Nonetheless, others and we have been successful in phenotyping some of the SNS outflow circuits to a variety of tissues. Perhaps to avoid this potential problem of virus-induced suppression of gene and protein expression, Voss-Andreae et al., (56) injected PRV into IBAT of MC4-R-GFP mutant mice, as noted above. In addition, Oldfield et al. (39) reported occasional high levels of colocalization of PRV with cocaine- and amphetamine-regulated transcript in the RCh and Arc (e.g., maximum = ∼87 and ∼79% of PRV infected cells) when PRV was injected in IBAT of laboratory rats. In that study, however, there was typically no more than ∼40–50% colocalizations (e.g., ∼32% of orexin B-positive cells in the lateral hypothalamus and ∼10–15% oxytocin-positive cells in the PVH, ∼44% proopiomelanocortin-positive cells in RCh).
We found PRV + MC4-R mRNA (and PRV-only infected cells) in the caudal pole of the facial nucleus [cranial nerve (CN) VII], a CN known traditionally for the control of facial movements, taste, salivation, lacrimation, and general sensation from the external ear. This area, however, projects to the rostroventrolateral medulla (54), a site of SNS outflow to several tissues including IBAT (4, 13, 38, 56) and receives cold thermal inputs from the skin that in turn engage sympathetic outflow circuits to IBAT and heart via raphé-spinal projections (44). Regarding the colocalization and PRV-only labeled cells in the nucleus of the vagus (CN X), we addressed this issue several times previously (22, 23) and note that here, as well as in our previous work in WAT and BAT (e.g., 3, 4), that the dorsal motor nucleus of the vagus nerve labeling is on its peripheral edge unilateral to the sites of injection (4, 22, 53). Thus, this appears to reflect the lack of homogeneity of this classically known parasympathetic nucleus at its extremities. We also found that the hypoglossal nucleus (CN XII) had MC4-R + PRV-labeled and PRV-only infected cells. CN XII is known for control of the intrinsic muscles of the tongue and muscles associated with shoulder movement; however, CN XII also is involved in cardiovascular and especially respiratory circuits (35) involving the PVH, an area of high colocalization of PRV + MC4-R mRNA cells, as well as modulation by the ROb (41), another area of high colocalization. It is not difficult to envision coordination of respiration, cardiovascular and BAT temperature responses to changes in ambient temperature. Finally, in terms of the oculomotor nucleus (CN III), we have reported similar infections previously after injection of PRV into WAT (53). Although we are at a loss to explain how a site classically known for the control of eye movement and pupil constriction and accommodation could also be involved in BAT/temperature regulation. Based on the above findings, however, we choose to believe that these and other PRV-generated data teach us something new about neural circuitry; in this case an apparent role of CN III in IBAT function to some degree.
We chose the PVH as a representative site to test whether MC4-R agonism would increase IBAT temperature for several reasons. First, the PVH has been implicated in energy expenditure because electrical stimulation (20) or microinjection of glutamate increases IBAT temperature (1), although the ability of PVH stimulation to increase IBAT temperature or sympathetic activity to IBAT is not universally reported (e.g., 28, 47). Lesions of the PVH, however, tend to support the notion of a normally stimulatory circuit involving the PVH and ending in IBAT in that both whole animal oxygen consumption and colonic temperature are decreased after such lesions (17). In addition, because PVH lesions block cafeteria diet-induced increases in these measures, it has been concluded that diet-induced IBAT growth and increased IBAT thermogenic function are dependent on an intact PVH (17). Secondly, the PVH shows impressive PRV infection in most of its divisions after PRV is injected into IBAT in laboratory rats (13, 39), as well as in our Siberian hamsters (4). Finally, as shown in the present study, there is high colocalization of MC4-R mRNA with PRV infected cells, averaging for example ∼80% across all subdivisions of the PVH.
Our findings in the present study that MTII microinjected into the PVH increased IBAT temperature appear to differ with a transgenic mouse study where genetic reinsertion of the MC4-Rs into the PVH (and simultaneously medial amygdala) normalized the hyperphagia of the MC4-R knockout mice and did not elevate their oxygen consumption to normal wild-type levels (2). In addition, peripheral injection of MTII did not increase oxygen consumption to levels seen in their wild-type counterparts (2). The reason why MTII did not activate thermogenesis following the genetic reinsertion of MC4-Rs into the PVH in mice (2), whereas it convincingly stimulated thermogenesis when injected in the PVH in the Siberian hamsters in our hands remains unclear. The MTII-induced increase in IBAT temperature in the present study was not due to cannulae located in sites adjacent to the PVH that contained MC4-R + PRV labeled cells, because cannulae placement in the PVH, where more than ∼80% of PRV infected neurons were also labeled for MC4-R mRNA, was confirmed histologically. In addition, it would seem unlikely that the small volume (200 nl) injected into the PVH diffused to other population of PRV + MC4-R mRNA neurons. Moreover, it cannot be that the MTII injection diffused into the third ventricle to stimulate distant sites of SNS outflow neurons possessing MC4-Rs because doses of MTII equivalent or even somewhat larger doses purposefully injected into the third ventricle were insufficient to increase BAT temperature in the present study (Table 1). Thus, it is not easy to conceive how such an important and large clustering of MC4-R harboring neurons connected to IBAT would not ultimately activate thermogenesis once stimulated by the MC4-R agonist MTII. Therefore, the present results provide evidence that the PVH MC4-R containing SNS outflow neurons to IBAT has, at least in Siberian hamsters, the ability/capacity to stimulate BAT thermogenesis and hence energy expenditure, while demonstrating that this population is likely not the only one to be involved in such functions. Two alternative hypotheses for the discrepancies between the transgenic mouse study (2) and the present study could be a simple species difference (laboratory mice vs. Siberian hamsters) and/or incomplete genetic reinsertion of the MC4-R in the PVH using the Sim1-Cre transgenic method. We find the species differences-hypothesis unlikely, however, because we previously demonstrated that PRV injected into IBAT of Siberian hamsters or laboratory rats results in a similar central patterns of infection between these two species (4). In addition, although we found additional areas of PRV + MC4-R mRNA colocalizations compared with the transgenic mouse study (2), using nongenetic methodology in Siberian hamsters, we found colocalizations in all areas they reported. Thus, although not identical in terms of the additional areas of colocalization found in the present study compared with the transgenic study (2), the pattern of colocalizations was more similar than different in many ways. This too suggests that species differences are not the root of the discrepant findings of the effects of MTII on thermogenesis.
Additional support for a role of the melanocortins and MC3/4-Rs in IBAT thermogenesis comes from work conducted in normal laboratory rats and rats that receive a complete transection of the neuroaxis at the mesencephalic-diencephalic juncture severing all ascending and descending connections between the forebrain and hindbrain [i.e., chronic decerebrate (CD) rats; for a review, see Ref. 25]. In intact rats, third and fourth ventricularly administered MTII were equally effective at elevating IBAT UCP-1 mRNA expression, indicative of increases in thermogenesis, compared with their respective controls (57). The fourth ventricular MTII-induced rise in IBAT UCP-1 gene expression was mediated by sympathetic outflow to IBAT because surgical sympathetic denervation of IBAT blocked this effect (57). Fourth ventricular MTII in CDs, as with their neurologically intact controls, increased UCP-1 mRNA; thus, collectively, there appears to be sufficient caudal brain stem MC3/4-R-mediated sympathetic IBAT circuitry to affect UCP-1 gene expression and presumably IBAT temperature (57). More recently, it was found that both fourth ventricular and hindbrain parenchymal (medullary raphé) MTII injections increased body temperature, IBAT temperature, and heart rate and did so in both neurologically intact and CD rats, with enhanced responses for both routes of MTII administration in the CDs compared with intact rats (50). Collectively, the above data suggest that melanocortin receptors, most likely MC4-Rs, are involved not only in IBAT thermogenesis, but also in other thermogenic (body temperature) or related responses (heart rate) and that the control of IBAT thermogenesis seems to be via a widely distributed CNS system.
In summary, these data reinforce reports that in both human (e.g., 55, 59) and nonhuman animals (e.g., 29), the MC4-R is important in energy balance and, in this case, implicated in the energy expenditure component (BAT thermogenesis) of the energy balance equation.
Perspectives and Significance
There was an initial excitement concerning a significant role of BAT in energy expenditure via diet-induced thermogenesis (46) where increases in BAT temperature might significantly oppose storage of excess calories eaten, perhaps even in humans. With only clear evidence of significant BAT depots in human infants (33) or in adults only with pathologies such as pheochromocytoma (32), this excitement waned rapidly. Recently, however, two findings renewed interest in the potential for BAT to significantly contribute to energy expenditure in adult humans. The first is the ability of some white adipocytes to transdifferentiate into brown adipocytes, as evidenced by the presence of UCP-1 that is an exclusive property of brown adipocytes, under conditions of increased adrenergic stimulation (e.g., 26). That is, although the precise process by which this switch between white and brown adipocytes occurs is unknown, it is clear that such transdifferentiation occurs with chronic β-adrenoceptor stimulation (for a review, see Ref. 15), thereby giving some hope to increasing the population of brown adipocytes in humans therapeutically to oppose obesity. The second finding spurring renewed interest in a physiological function of BAT in humans is the presence of indisputable BAT depots in adult humans, as revealed accidentally through the use of fluorine-18 fluorodeoxyglucose in identifying tumors. Using this clinical procedure, clinicians found BAT depots occurring in the supraclavicular, superior mediastinal, paravertebral, and suprarenal/perinephric regions (30; for a review, see Ref. 36). These findings coupled with report of reduced lipid oxidation of MC4-R deficient humans compared with their obese controls after a fast (37), as indicated by a higher respiratory quotient, implicate a role of the melanocortins in energy expenditure in human obesity perhaps, at least to some extent, via the SNS innervation of BAT.
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Research Grant R01-DK-35254 to T. J. Bartness and to Viral Tract Tracing Core through the Science Technology Center Program of the National Science Foundation under agreement No. IBN-9876754.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Amir S Stimulation of the paraventricular nucleus with glutamate activates interscapular brown adipose tissue thermogenesis in rats. Brain Res 508: 152–155, 1990. [DOI] [PubMed] [Google Scholar]
- 2.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123: 493–505, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Bamshad M, Aoki VT, Adkison MG, Warren WS, Bartness TJ. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am J Physiol Regul Integr Comp Physiol 275: R291–R299, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 276: R1569–R1578, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Bartness TJ, Song CK. Brain-adipose tissue neural crosstalk. Physiol Behav 91: :343–351, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartness TJ, Song CK. Sympathetic and sensory innervation of white adipose tissue. J Lipid Res 48: 1655–1672, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Bartness TJ, Song CK, Demas GE. Central nervous system innervation of brown adipose tissue. In: Adipose Tissue, edited by Klaus S. Georgetown, TX: Landes Bioscience, 2001, p. 162–200.
- 8.Berthoud HR, Patterson LM, Sutton GM, Morrison C, Zheng H. Orexin inputs to caudal raphé neurons involved in thermal, cardiovascular, and gastrointestinal regulation. Histochem Cell Biol 123: 147–156, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Billington CJ, Briggs JE, Harker S, Grace M, Levine AS. Neuropeptide Y in hypothalamic paraventricular nucleus: a center coordinating energy metabolism. Am J Physiol Regul Integr Comp Physiol 266: R1765–R1770, 1994. [DOI] [PubMed] [Google Scholar]
- 10.Bowers RR, Festuccia WTL, Song CK, Shi H, Migliorini RH, Bartness TJ. Sympathetic innervation of white adipose tissue and its regulation of fat cell number. Am J Physiol Regul Integr Comp Physiol 286: R1167–R1175, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Brito MN, Brito NA, Baro DJ, Song CK, Bartness TJ. Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology 148: 5339–53347, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Brito NA, Brito MN, Bartness TJ. Differential sympathetic drive to adipose tissues after food deprivation, cold exposure or glucoprivation. Am J Physiol Regul Integr Comp Physiol 294: R1445–R1452, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol 460: 303–326, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Card JP Practical considerations for the use of pseudorabies virus in transneuronal studies of neural circuitry. Neurosci Biobehav Rev 22: 685–694, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Cinti S The role of brown adipose tissue in human obesity. Nutr Metab Cardiovasc Dis 16: 569–574, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Collins S, Migliorini RH, Bartness TJ. Mechanisms controlling adipose tissue metabolism by the sympathetic nervous system: anatomical and molecular aspects. In: Handbook of Contemporary Neuropharmacology, edited by Sibley D, Hanin I, Kuhar M, and Skolnick P. New York: Wiley, 2007, p. 785–814.
- 17.De LB, Monda M, Amaro S, Pellicano MP, Cioffi LA. Lack of diet-induced thermogenesis following lesions of paraventricular nucleus in rats. Physiol Behav 46: 685–691, 1989. [DOI] [PubMed] [Google Scholar]
- 18.Demas GE, Bowers RR, Bartness TJ, Gettys TW. Photoperiodic regulation of gene expression in brown and white adipose tissue of Siberian hamsters (Phodopus sungorus). Am J Physiol Regul Integr Comp Physiol 282: R114–R121, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Ellacott KL, Cone RD. The central melanocortin system and the integration of short- and long-term regulators of energy homeostasis. Recent Prog Horm Res 59: 395–408, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Freeman PH, Wellman PJ. Brown adipose tissue thermogenesis induced by low level electrical stimulation of hypothalamus in rats. Brain Res Bull 18: 7–11, 1987. [DOI] [PubMed] [Google Scholar]
- 21.Giordano A, Frontini A, Castellucci M, Cinti S. Presence and distribution of cholinergic nerves in rat mediastinal brown adipose tissue. J Histochem Cytochem 52: 923–930, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Giordano A, Song CK, Bowers RR, Ehlen JC, Frontini A, Cinti S, Bartness TJ. White adipose tissue lacks significant vagal innervation and immunohistochemical evidence of parasympathetic innervation. Am J Physiol Regul Integr Comp Physiol 291: R1243–R1255, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Giordano A, Song CK, Bowers RR, Ehlen JC, Frontini A, Cinti S, Bartness TJ. No sympathy for the claim of parasympathetic innervation of white adipose tissue. Am J Physiol Regul Integr Comp Physiol 293: R550–R552, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Grill HJ, Ginsberg AB, Seeley RJ, Kaplan JM. Brainstem application of melanocortin receptor ligands produces long-lasting effects on feeding and body weight. J Neurosci 18: 10128–10135, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grill HJ, Kaplan JM. The neuroanatomical axis for control of energy balance. Front Neuroendocrinol 23: 2–40, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol 279: C670–C681, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Hollopeter G, Erickson JC, Seeley RJ, Marsh DJ, Palmiter RD. Response of neuropeptide Y-deficient mice to feeding effectors. Regul Pept 75–76: 383–389, 1998. [DOI] [PubMed]
- 28.Holt SJ, Wheal HV, York DA. Response of brown adipose tissue to electrical stimulation of hypothalamus centres in intact and adrenalectomized Zucker rats. Neurosci Lett 84: 63–67, 1988. [DOI] [PubMed] [Google Scholar]
- 29.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88: 131–141, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Kim S, Krynyckyi BR, Machac J, Kim CK. Concomitant paravertebral FDG uptake helps differentiate supraclavicular and suprarenal brown fat uptake from malignant uptake when CT coregistration is not available. Clin Nucl Med 31: 127–130, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol 457: 213–235, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Lean ME, James WP, Jennings G, Trayhurn P. Brown adipose tissue in patients with phaeochromocytoma. Int J Obes 10: 219–227, 1986. [PubMed] [Google Scholar]
- 33.Lean MEJ, James WPT. Brown adipose tissue in man. In: Brown Adipose Tissue, edited by Trayhurn P and Nicholls DG. London: Edward Arnold, 1986, p. 339–365.
- 34.Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM, Elmquist JK. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci 23: 7143–7154, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mack SO, Wu M, Kc P, Haxhiu MA. Stimulation of the hypothalamic paraventricular nucleus modulates cardiorespiratory responses via oxytocinergic innervation of neurons in pre-Botzinger complex. J Appl Physiol 102: 189–199, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 293: E444–E452, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Nogueiras R, Wiedmer P, Perez-Tilve D, Veyrat-Durebex C, Keogh JM, Sutton GM, Pfluger PT, Castaneda TR, Neschen S, Hofmann SM, Howles PN, Morgan DA, Benoit SC, Szanto I, Schrott B, Schurmann A, Joost HG, Hammond C, Hui DY, Woods SC, Rahmouni K, Butler AA, Farooqi IS, O'Rahilly S, Rohner-Jeanrenaud F, Tschop MH. The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest 117: 3475–3488, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oldfield BJ, Allen AM, Davern P, Giles ME, Owens NC. Lateral hypothalamic 'command neurons' with axonal projections to regions involved in both feeding and thermogenesis. Eur J Neurosci 25: 2404–2412, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Oldfield BJ, Giles ME, Watson A, Anderson C, Colvill LM, McKinley MJ. The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience 110: 515–526, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. New York: Academic, 2007.
- 41.Peever JH, Necakov A, Duffin J. Nucleus raphé obscurus modulates hypoglossal output of neonatal rat in vitro transverse brain stem slices. J Appl Physiol 90: 269–279, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Pritchard LE, Turnbull AV, White A. Pro-opiomelanocortin processing in the hypothalamus: impact on melanocortin signalling and obesity. J Endocrinol 172: 411–421, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Raposinho PD, White RB, Aubert ML. The melanocortin agonist melanotan-II reduces the orexigenic and adipogenic effects of neuropeptide Y (NPY) but does not affect the NPY-driven suppressive effects on the gonadotropic and somatotropic axes in the male rat. J Neuroendocrinol 15: 173–181, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Rathner JA, Owens NC, McAllen RM. Cold-activated raphé-spinal neurons in rats. J Physiol 535: 841–854, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richard D Energy expenditure: a critical determinant of energy balance with key hypothalamic controls. Minerva Endocrinol 32: 173–183, 2007. [PubMed] [Google Scholar]
- 46.Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature 281: 31–35, 1979. [DOI] [PubMed] [Google Scholar]
- 47.Saito M, Minokoshi Y, Shimazu T. Accelerated norepinephrine turnover in peripheral tissues after ventromedial hypothalamic stimulation in rats. Brain Res 481: 298–303, 1989. [DOI] [PubMed] [Google Scholar]
- 48.Shi H, Bartness TJ. Neurochemical phenotype of sympathetic nervous system outflow from brain to white fat. Brain Res Bull 54: 375–385, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Shi H, Bowers RR, Bartness TJ. Norepinephrine turnover in brown and white adipose tissue after partial lipectomy. Physiol Behav 81: 535–543, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Skibicka KP, Grill HJ. Energetic responses are triggered by caudal brainstem melanocortin receptor stimulation and mediated by local sympathetic effector circuits. Endocrinology. In Press. [DOI] [PMC free article] [PubMed]
- 51.Song CK, Bartness TJ. CNS sympathetic outflow neurons to white fat that express melatonin receptors may mediate seasonal adiposity. Am J Physiol Regul Integr Comp Physiol 281: R666–R672, 2001. [DOI] [PubMed] [Google Scholar]
- 52.Song CK, Enquist LW, Bartness TJ. New developments in viral tracings of neural circuits. Virus Res 11: 235–249, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Song CK, Jackson RX, Harris RBS, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA is expressed in sympathetic nervous system outflow neurons to white adipose tissue. Am J Physiol Regul Integr Comp Physiol 289: R1467–R1476, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Sun QJ, Minson J, Llewellyn-Smith IJ, Arnolda L, Chalmers J, Pilowsky P. Botzinger neurons project towards bulbospinal neurons in the rostral ventrolateral medulla of the rat. J Comp Neurol 388: 23–31, 1997. [DOI] [PubMed] [Google Scholar]
- 55.Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet 20: 113–114, 1998. [DOI] [PubMed] [Google Scholar]
- 56.Voss-Andreae A, Murphy JG, Ellacott KL, Stuart RC, Nillni EA, Cone RD, Fan W. Role of the central melanocortin circuitry in adaptive thermogenesis of brown adipose tissue. Endocrinology 148: 1550–1560, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Williams DL, Bowers RR, Bartness TJ, Kaplan JM, Grill HJ. Brainstem melanocortin 3/4 receptor stimulation increases uncoupling protein gene expression in brown fat. Endocrinology 144: 4692–4697, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Yasuda T, Masaki T, Kakuma T, Yoshimatsu H. Hypothalamic melanocortin system regulates sympathetic nerve activity in brown adipose tissue. Exp Biol Med (Maywood) 229: 235–239, 2004. [DOI] [PubMed] [Google Scholar]
- 59.Yeo GS, Lank EJ, Farooqi IS, Keogh J, Challis BG, O'Rahilly S. Mutations in the human melanocortin-4 receptor gene associated with severe familial obesity disrupts receptor function through multiple molecular mechanisms. Hum Mol Genet 12: 561–574, 2003. [DOI] [PubMed] [Google Scholar]
- 60.Yoshimatsu H, Egawa M, Bray GA. Sympathetic nerve activity after discrete hypothalamic injections of l-glutamate. Brain Res 601: 121–128, 1993. [DOI] [PubMed] [Google Scholar]