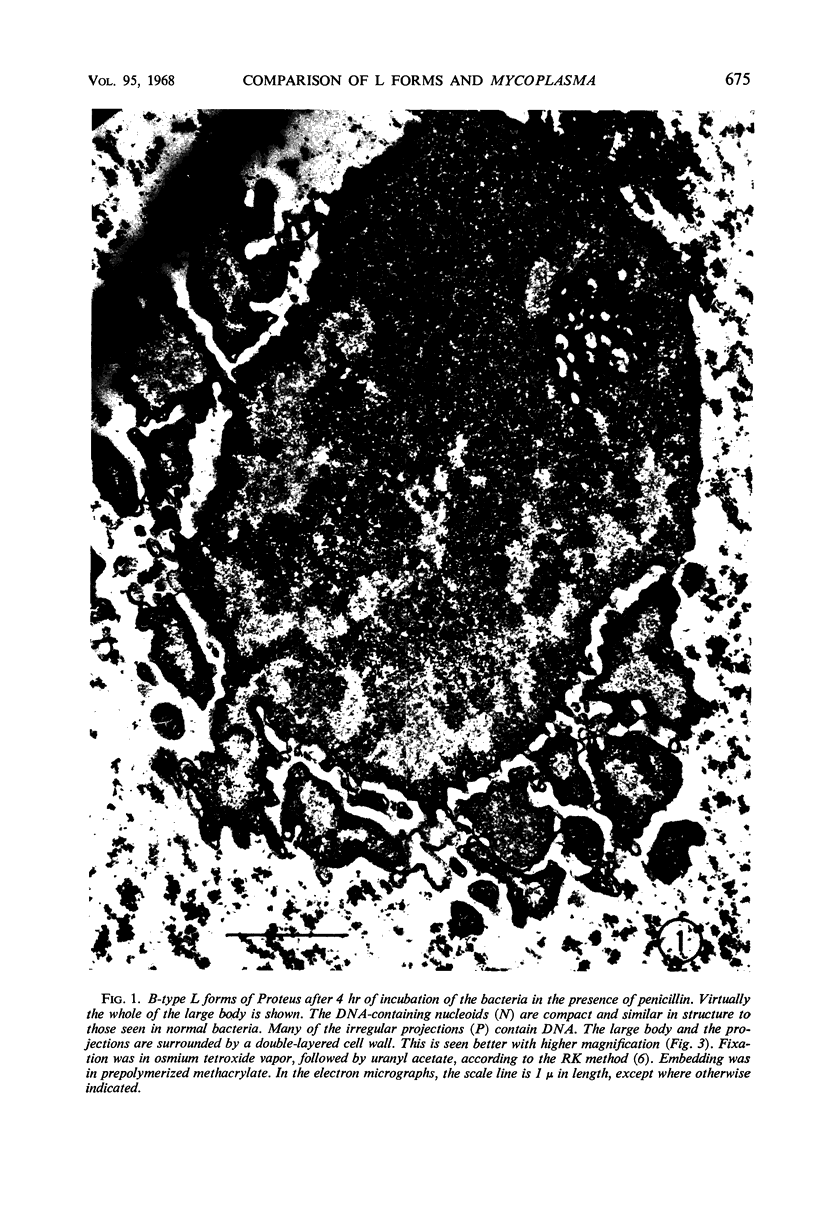
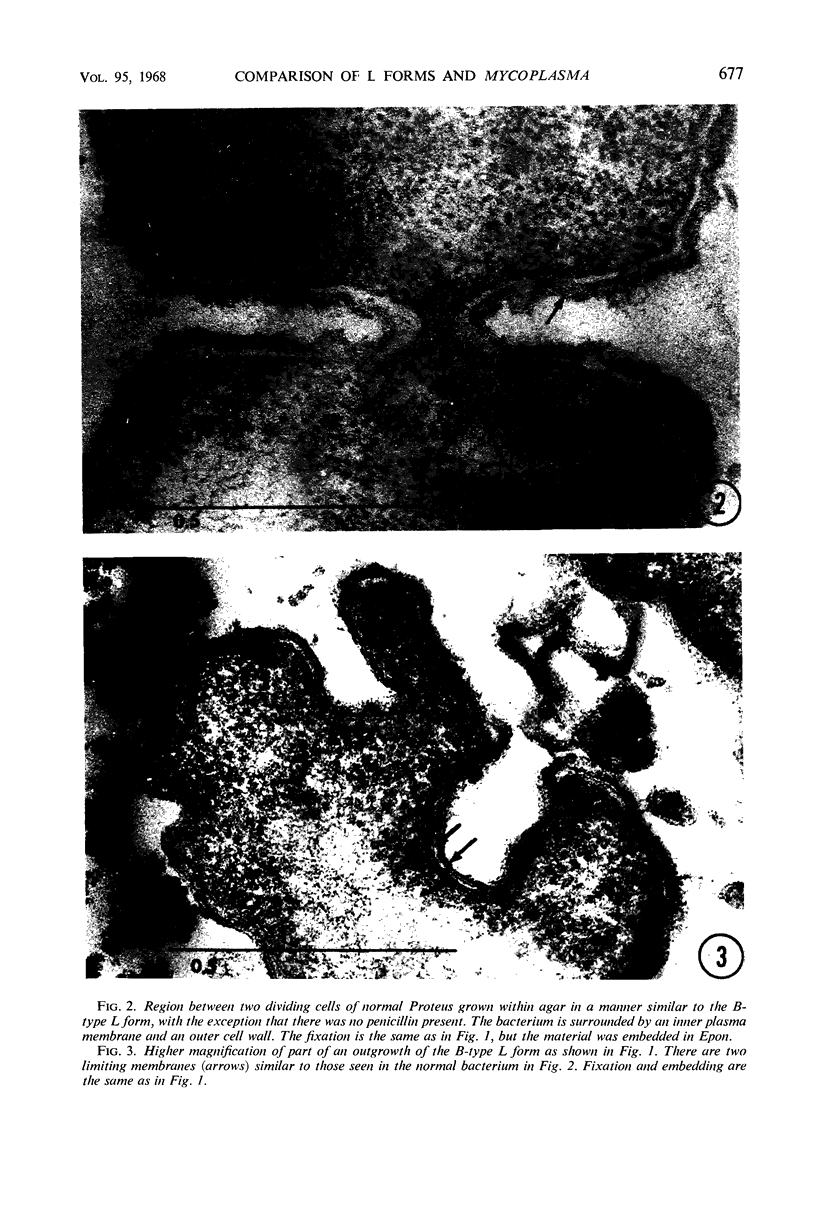
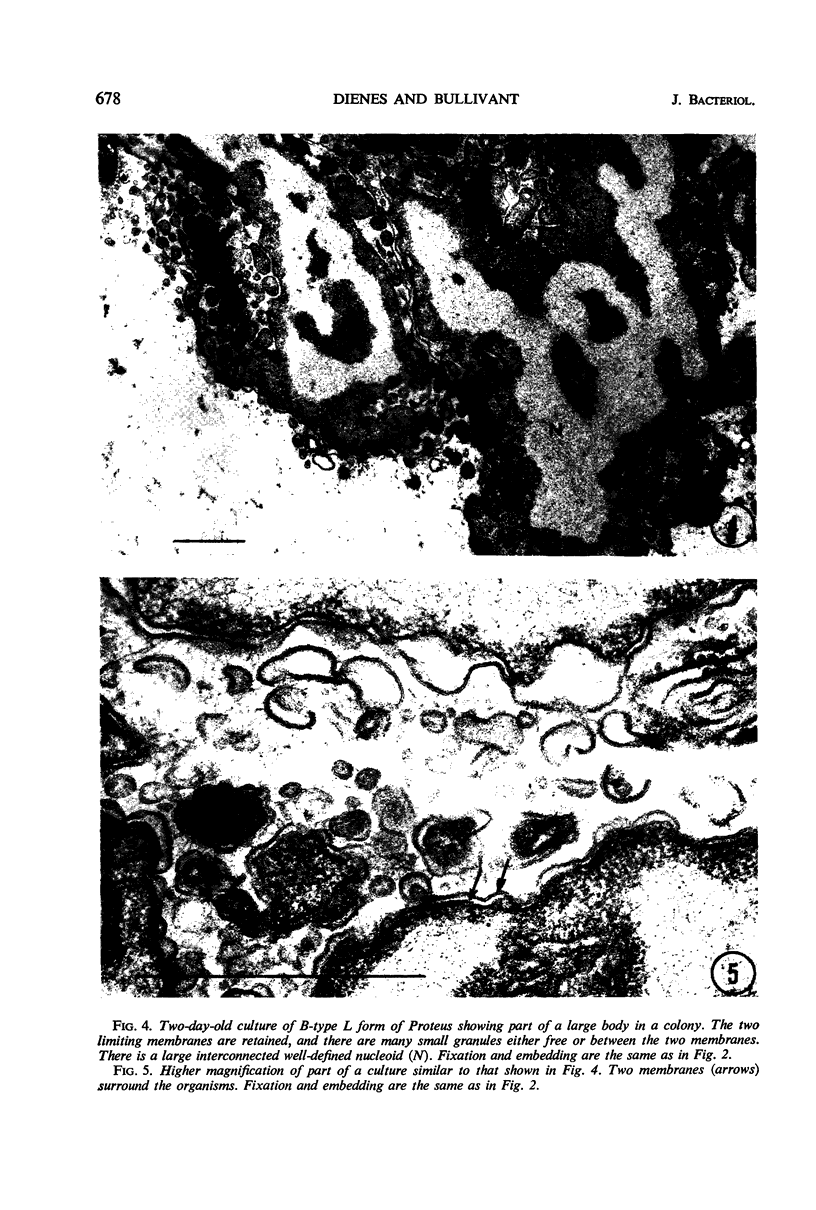
Abstract
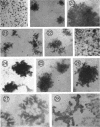
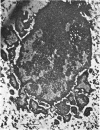
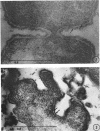
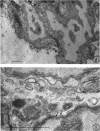
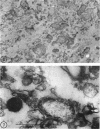
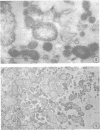
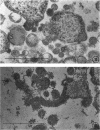
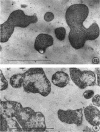
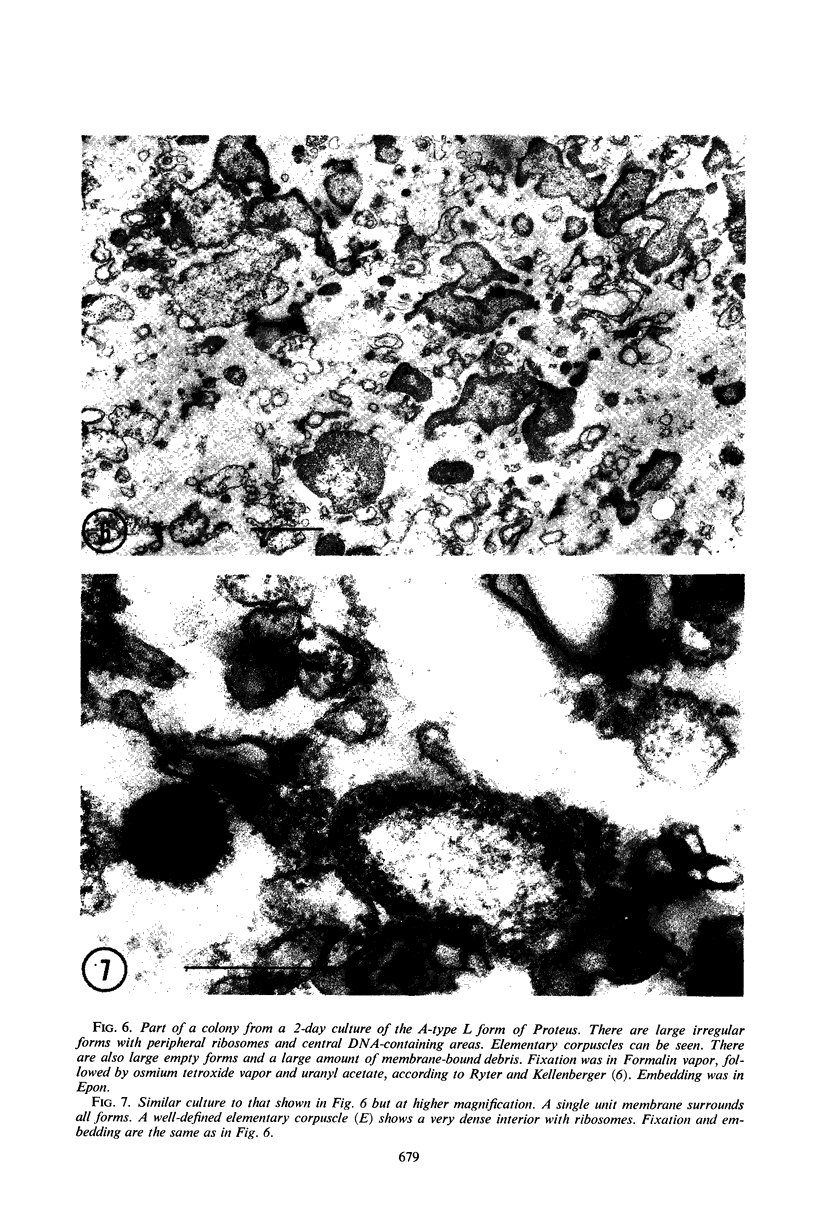
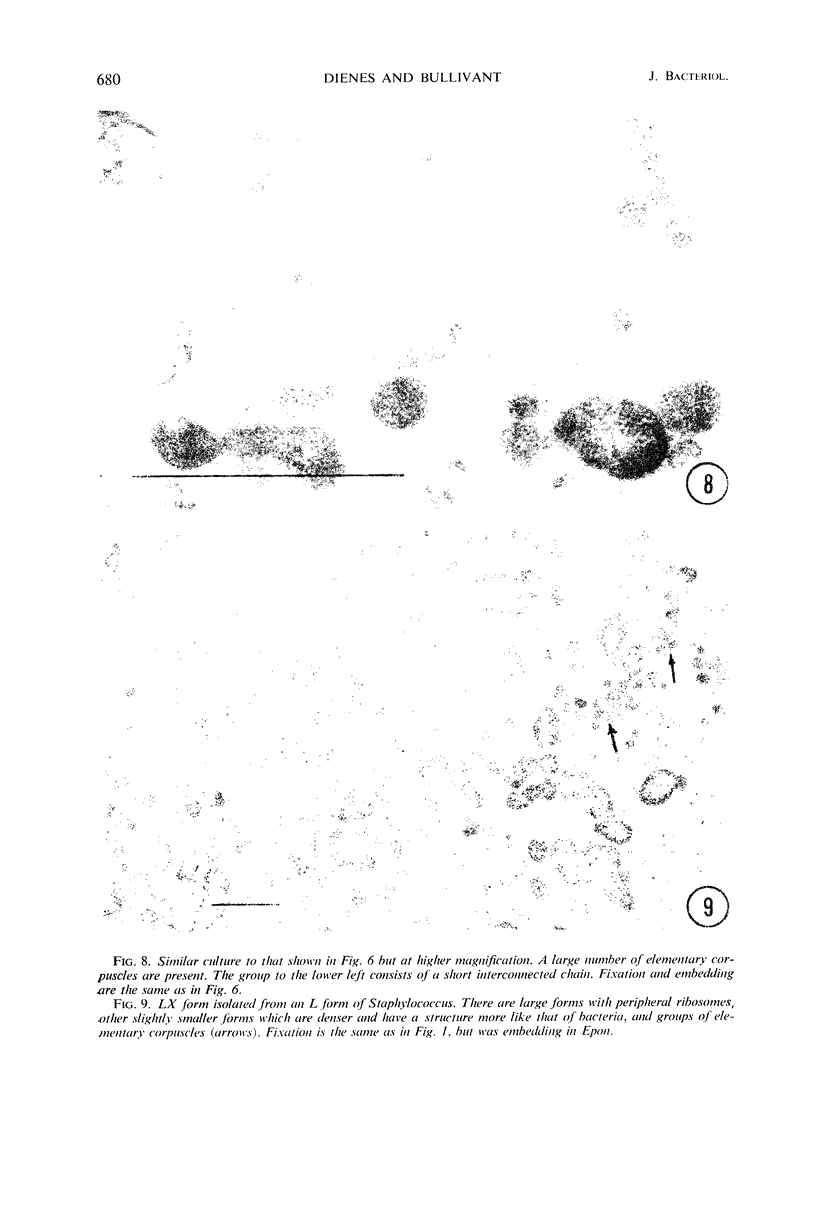
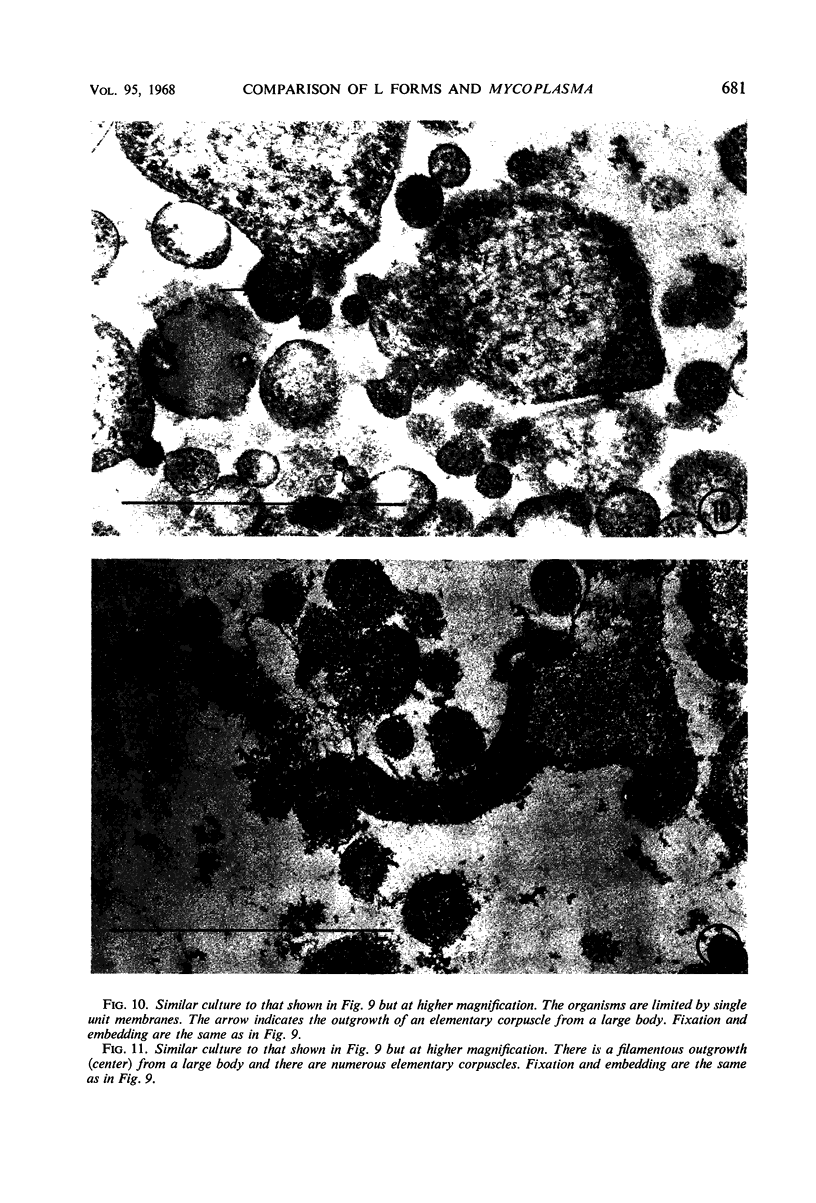
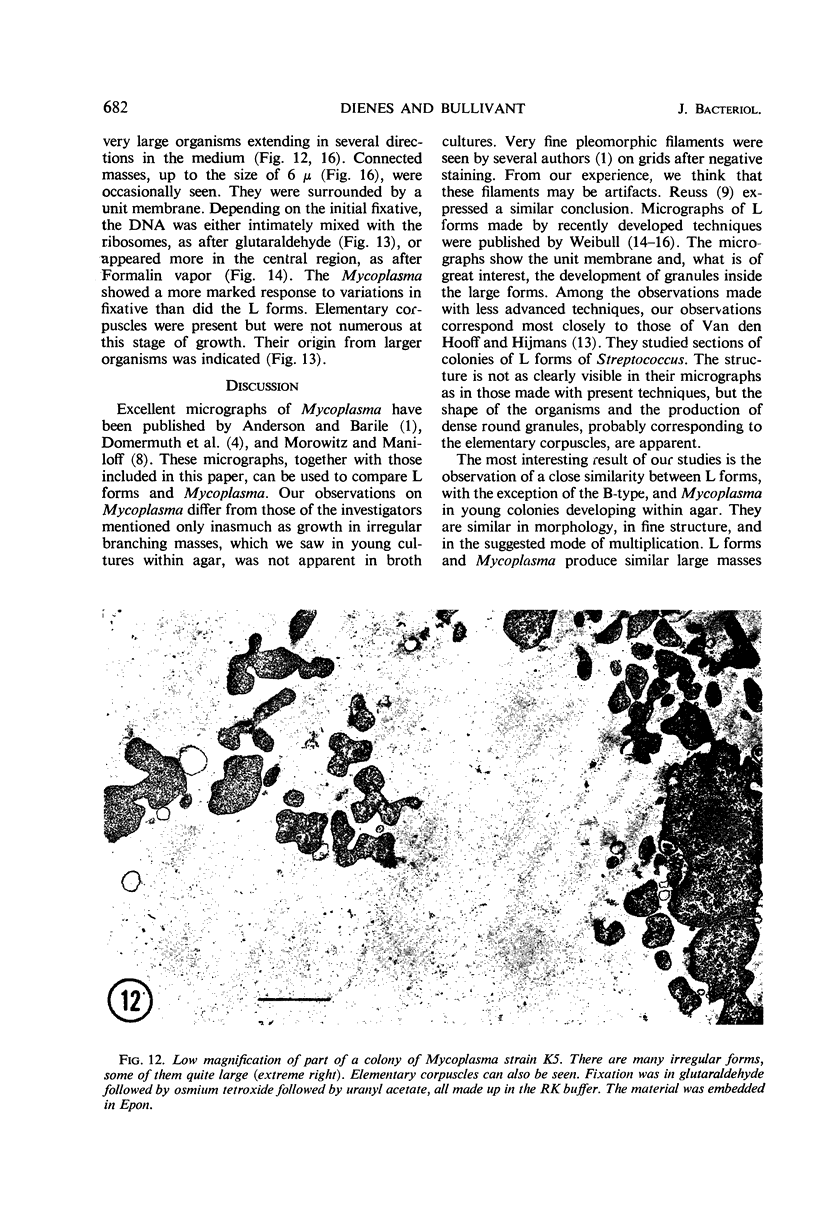
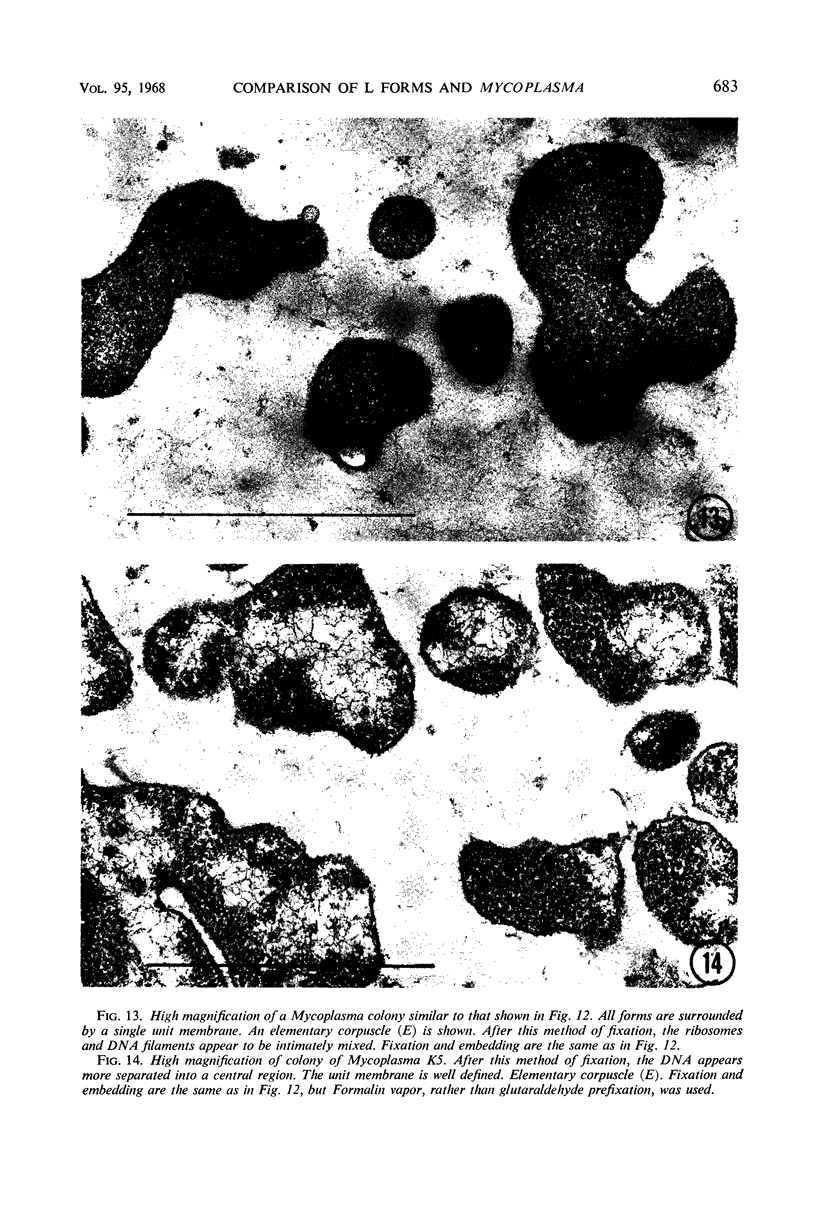
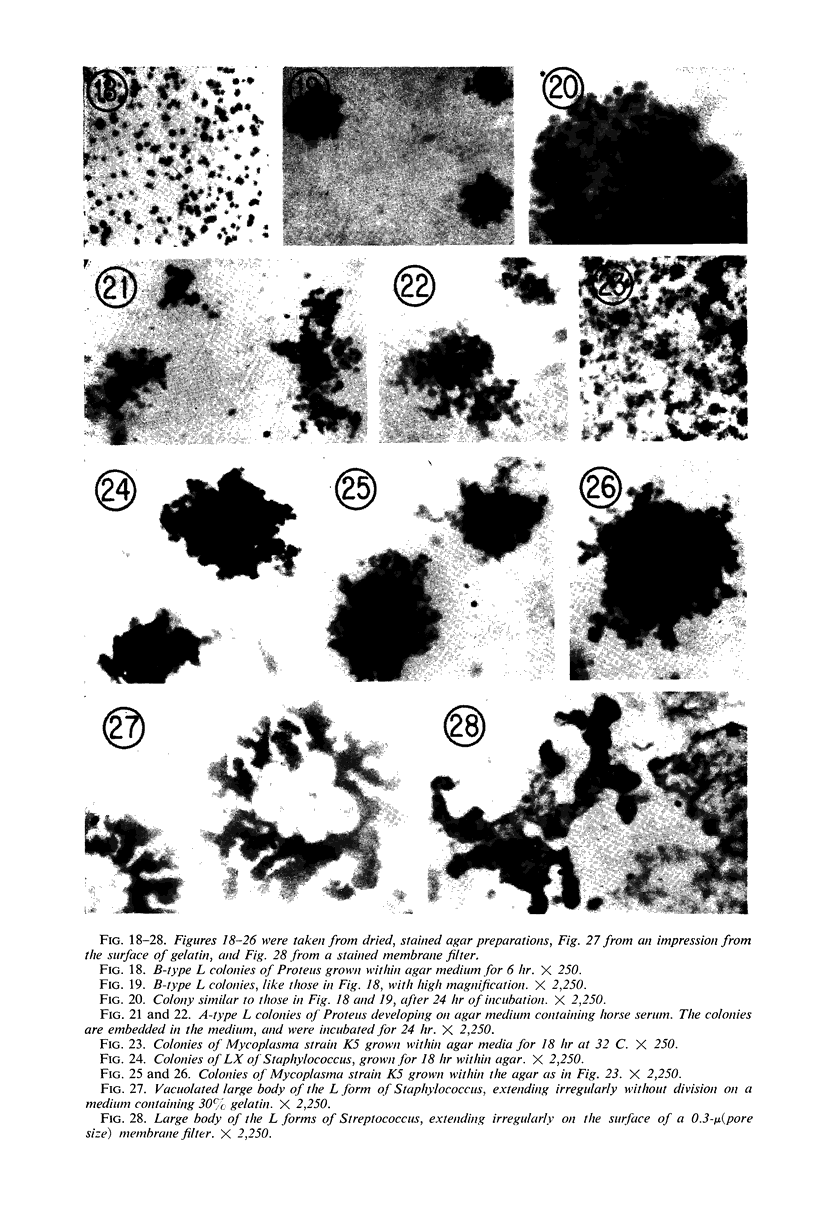
Representative electron micrographs, from the study of eight strains of L forms and one strain of Mycoplasma, are presented. A- and B-type L forms were derived from two strains of Proteus, two other L forms were derived from a diphtheroid and from a staphylococcus strain, and two strains (designated as LX) were isolated from L forms derived from a group A β-hemolytic streptococcus and from a staphylococcus. The Mycoplasma strain was isolated from goats. Sections were made of young colonies grown within agar and from parts of surface colonies embedded in the agar. B-type L colonies of Proteus were produced by inoculation of bacteria into media containing penicillin. The large bodies developing from the bacteria and the organisms in B-type L colonies of Proteus, like the parent bacteria, had a cell wall consisting of a plasma membrane and an outer cell wall. The loss of rigidity in the cell wall indicated an alteration in its structure. The A-type L cultures of Proteus consisted of irregular branching masses extending in several directions, of small dense organisms corresponding to the elementary corpuscles present in cultures of Mycoplasma, and of intermediary forms. In contrast to the B-type, all organisms in the A-type colonies were surrounded by a single unit membrane corresponding to the plasma membrane of bacteria. The structures inside the cell membrane, both in the A- and B-type, seemed to correspond to the structure of the parent bacteria, which contained ribosomes and threads of DNA. The elementary corpuscles formed chains and filaments, and, apparently, these corpuscles took part in the multiplication by gradual enlargement. The organisms seen in the cultures of all L forms and Mycoplasma studied, except in the B-type L forms of Proteus, corresponded in size, shape, and structure, as well as in the development of elementary corpuscles, to the organisms in the A-type L form of Proteus. In contrast to the spherical organisms usually seen in broth cultures, the organisms in young cultures of Mycoplasma, which were grown within the agar, were similar in morphology, as well as in the discernible structure of the organisms, to L forms. Significant morphological and structural differences were not apparent between the L forms and Mycoplasma (in cultures grown within agar media) under the conditions of this investigation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. R., Barile M. F. Ultrastructure of Mycoplasma hominis. J Bacteriol. 1965 Jul;90(1):180–192. doi: 10.1128/jb.90.1.180-192.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOMERMUTH C. H., NIELSEN M. H., FREUNDT E. A., BIRCH-ANDERSEN A. ULTRASTRUCTURE OF MYCOPLASMA SPECIES. J Bacteriol. 1964 Sep;88:727–744. doi: 10.1128/jb.88.3.727-744.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienes L. Morphology and reproductive processes of the L forms of bacteria. I. Streptococci and staphylocci. J Bacteriol. 1967 Feb;93(2):693–702. doi: 10.1128/jb.93.2.693-702.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienes L. THE DEVELOPMENT OF PROTEUS CULTURES IN THE PRESENCE OF PENICILLIN. J Bacteriol. 1949 May;57(5):529–546. doi: 10.1128/jb.57.5.529-546.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morowitz H. J., Maniloff J. Analysis of the life cycle of Mycoplasma gallisepticum. J Bacteriol. 1966 Apr;91(4):1638–1644. doi: 10.1128/jb.91.4.1638-1644.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A., LANDMAN O. E. ELECTRON MICROSCOPE STUDY OF THE RELATIONSHIP BETWEEN MESOSOME LOSS AND THE STABLE L STATE (OR PROTOPLAST STATE) IN BACILLUS SUBTILIS. J Bacteriol. 1964 Aug;88:457–467. doi: 10.1128/jb.88.2.457-467.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss K. Influence of fixation on gross morphology of Mycoplasma. J Bacteriol. 1967 Jan;93(1):490–492. doi: 10.1128/jb.93.1.490-492.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORSSON K. G., WEIBULL C. Studies on the structure of bacterial L forms, protoplasts and protoplast-like bodies. J Ultrastruct Res. 1958 Aug;1(4):412–427. doi: 10.1016/s0022-5320(58)90011-x. [DOI] [PubMed] [Google Scholar]
- WEIBULL C., LUNDIN B. M. Morphology of pleuropneumonia-like organisms and bacterial L forms grown in liquid media. J Bacteriol. 1963 Feb;85:440–445. doi: 10.1128/jb.85.2.440-445.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibull C., Bickel W. D., Haskins W. T., Milner K. C., Ribi E. Chemical, biological, and structural properties of stable Proteus L forms and their parent bacteria. J Bacteriol. 1967 Mar;93(3):1143–1159. doi: 10.1128/jb.93.3.1143-1159.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibull C. Structure of bacterial L forms and their parent bacteria. J Bacteriol. 1965 Nov;90(5):1467–1480. doi: 10.1128/jb.90.5.1467-1480.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]