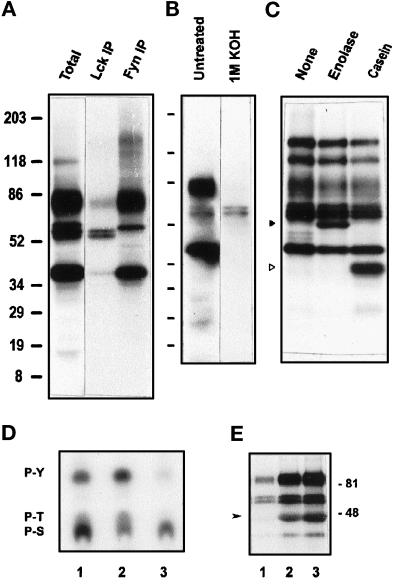
Figure 4.
GPI-enriched membranes harbor both Tyr and Ser/Thr kinase activities. (A) Immunoprecipitation of Lck and Fyn kinases. After the kinase assay, [32P]ATP on TX-100–isolated, GPI-enriched membranes (as in Figure 2C, lane 2), phosphorylated proteins were solubilized in SDS and immunoprecipitated with anti-Lck or anti-Fyn antibodies. The eluted phosphoproteins were separated by SDS-PAGE and detected by autoradiography after 4 d of exposure. (B) Base hydrolysis of the in vitro–phosphorylated proteins of GPI domains. After the kinase assay on TX-100–isolated, GPI-enriched membranes, the in vitro–phosphorylated proteins separated in 5–20% gels were transferred to a PVDF membrane and exposed to x-ray film for 1 d. Subsequently, the membrane was treated with 1 M KOH at 55°C for 1.5 h, rinsed, and exposed again for 15 d to reveal phosphorylations resistant to alkaline pH. (C) Phosphorylation of exogenous protein substrates by PTKs in GPI-enriched membranes. TX-100–treated GPI-enriched membranes (0.5 ml) were subjected to in vitro kinase assay as in Figure 2C, in the absence or presence of 5 μg of enolase or α-casein. Phosphoproteins separated in 5–20% gradient gels were revealed by autoradiography after overnight exposure. Phosphorylated enolase and α-casein are indicated by solid and open triangles, respectively. (D) Phosphoamino acid analysis of the major phosphoprotein bands. After in vitro kinase assay on TX-100–treated GPI-enriched membranes, the proteins were transferred onto a PVDF membrane and autoradiographed. Labeled bands corresponding to 80- to 85-kDa (lane 1), 56–59 Src kinase region (lane 2), and 40-kDa (lane 3) phosphoproteins were cut and acid hydrolyzed, and the phosphoamino acids were separated by TLC as described in MATERIALS AND METHODS. (E) Inhibition of phosphotransferase activities by genistein. TX-100–treated GPI-enriched membranes suspended in kinase buffer were incubated with 100 mM (lane 1) or 10 mM (lane 2) genistein or DMSO vehicle (lane 3) for 15 min at room temperature before the assay.