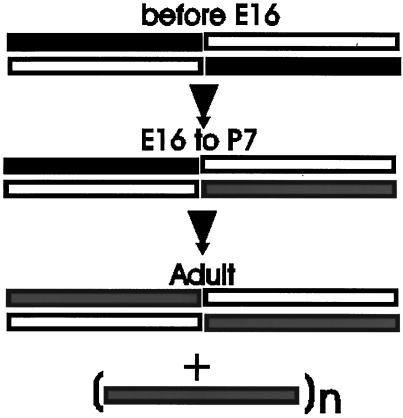
Figure 10.
Proposed model of changes in spectrin during skeletal muscle development. Open bar, α-fodrin; solid bar, β-fodrin; shaded bar, muscle β-spectrin. Our results can most easily be explained by a progressive change in the composition of spectrin tetramers as muscle fibers develop. Specifically, we propose that embryonic myoblasts and early myotubes contain tetramers of α- and β-fodrin (Weed, 1996), but that mixed tetramers containing these two subunits together with muscle β-spectrin begin to form with the onset of myogenesis and persist through early postnatal life. Adult muscle fibers contain heteromers composed of only α-fodrin and β-spectrin as well as β-spectrin that assembles at the sarcolemma without α-fodrin (Porter et al., 1997); the state of oligomerization of the latter population of β-spectrin is still unknown. This model does not include the proteins that bind to spectrin (e.g., actin, adducin, ankyrin, band 4.1) or other members of the spectrin superfamily that have not yet been identified, and so it may need to be revised when the associations of these proteins with spectrin are studied in mature and developing muscle fibers.