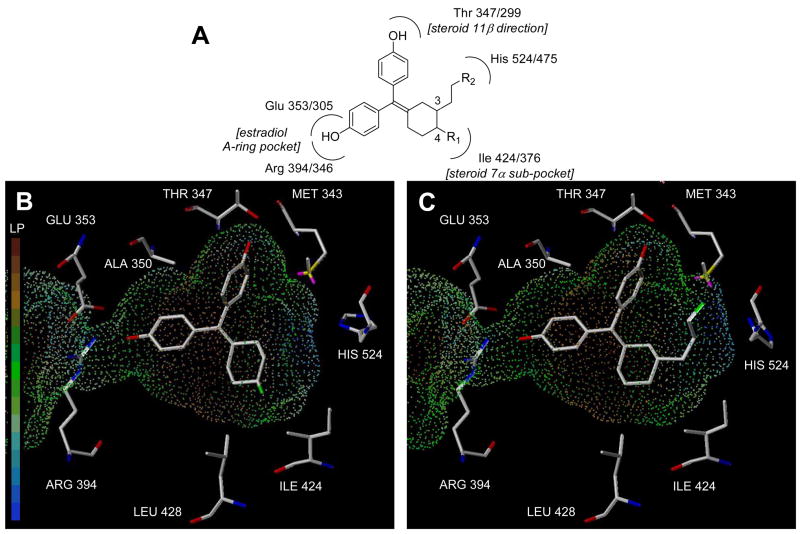
Figure 3.
Schematic and computational model of substituted cyclofenil interaction with the ligand binding pocket of the estrogen receptor, ERα. Panel A. Schematic picture of the interactions of the phenolic hydroxyls and the C3 and C4 cyclofenil substituents with residues in the ligand binding pocket. ERα residue numbers precede ERβ residue numbers. Panel B. Representative computational model of 4-fluorocyclofenil (20) in the ligand binding pocket of ERα. The hydrophobic residues near the C4 position are indicated. The surface shown is the ERα binding pocket displayed as dots mapped with the lipophilic potential. Residues identities are for ERα. Panel C. Representative computational model of 3-(2-fluoropropyl)cyclofenil (37c) in the ligand binding pocket of ERα. The surface is the same as Panel B. Residues identities are for ERα.