Scheme 2. Syntheses of cyclohexanones.
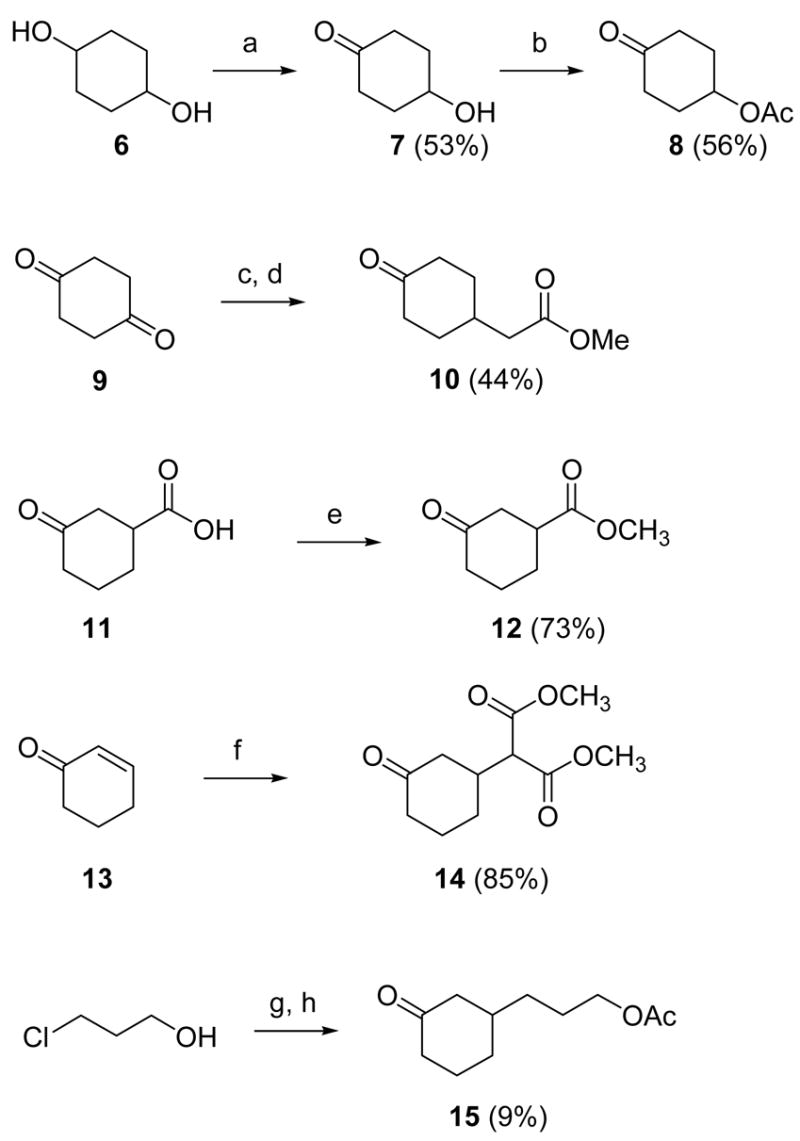
Reagents: (a) Jones reagent, acetone, 0 °C-rt, 45 min; (b) Ac2O, pyridine, rt, 24 h; (c) methyl (triphenylphosphoranylidene)acetate, toluene, 110 °C, 8 h; (d) Pd/C, H2, EtOH, rt, 6 h; (e) SOCl2, MeOH, rt, 3 h; (f) dimethyl malonate, NaOEt (cat.), rt, 2 h; (g) i. MeMgCl, THF, −78 °C-rt, 20 min; ii. Mg, 1,2-dibromoethane, THF, rt-90 °C, 2 h; iii. 13, CuBr, THF, −78 °C-0 °C, 1 h; (h) Ac2O, pyridine, CH2Cl2, rt, 18 h.
