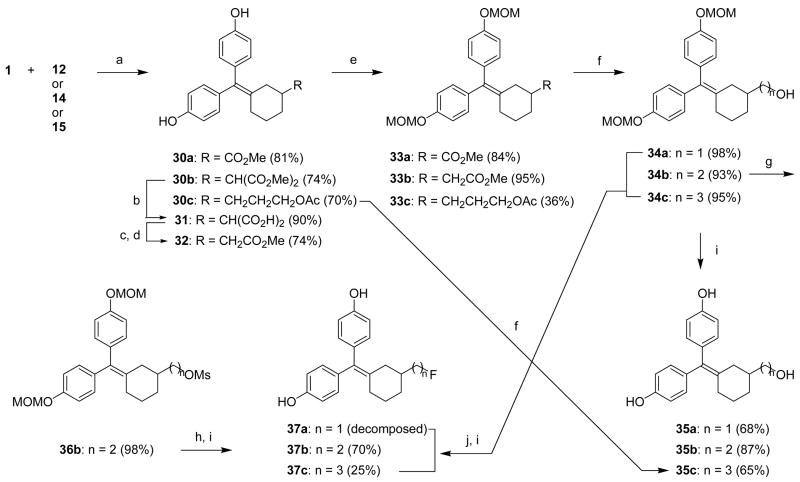
Scheme 5.
Reagents: (a) TiCl4, Zn, THF, reflux, 4 h; (b) 2 M NaOH, MeOH, reflux, 2 h; (c) diglyme, 160 °C, 1 h; (d) SOCl2, MeOH, rt, 90 min; (e) methoxymethyl chloride, NaH, DMF, 0 °C-rt, 1 h; (f) i. in case of 33a,b, LAH (1 M solution in THF), THF, 0 °C-rt, 1 h, ii. in case of 30c and 33c, K2CO3, MeOH:H2O (5:1), rt, 12 h; (g) methanesulfonyl anhydride, TEA, CH2Cl2, 0 °C-rt, 1 h; (h) CsF, H2O,1-butyl-3-methylimidazolium tetrafluoroborate ([bmim][BF4]), acetonitrile, 100 °C, 2 h; (i) HCl, MeOH, rt, 12 h; (j) DAST, CH2Cl2, −78 °C-rt, 1 h.