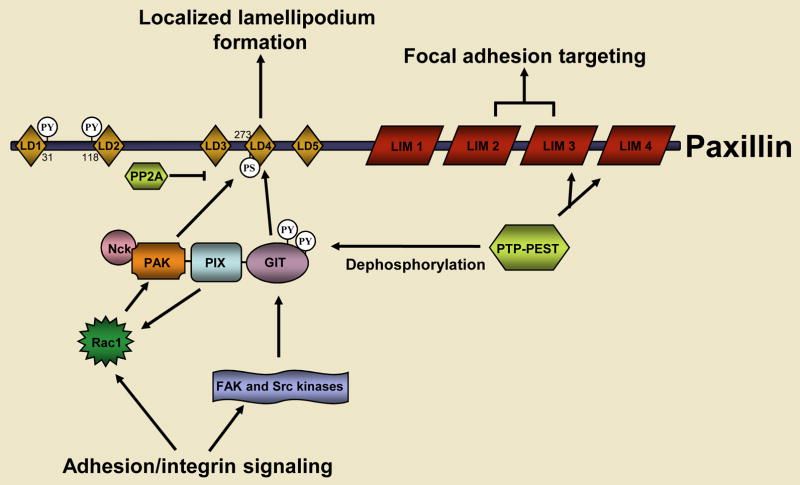
Fig. 3. The coordination of Rac signaling by the paxillin LD4 motif.
Paxillin is one of the earliest proteins that is recruited to nascent focal adhesions and is necessary for the turnover of focal adhesions during cell migration. The engagement of integrins with the extracellular matrix results in the localized activation of Rac and the Src and FAK tyrosine kinases. Together, Rac, Src and FAK promote the recruitment of the GIT-PIX-PAK-NCK complex to focal adhesions by means of an interaction between the paxillin binding subdomain 2 (PBS2) of GIT2 and the paxillin LD4 motif. Rac mediates this process by binding to and activating its effector PAK, which stimulates a multi-step conformational remodeling of the GIT-PIX-PAK-NCK complex and the PAK-dependent phosphorylation of the paxillin LD4 motif at S273. Src-FAK-dependent phosphorylation of GIT is also necessary for its binding to paxillin. The Rac GEF activity of PIX might function in a feed-forward loop to further promote localized Rac signaling to PAK at the cell’s leading edge. The recruitment of the GIT-PIX-PAK-NCK complex to paxillin also serves as a termination signal for Rac activity. This is also regulated at multiple levels, including the PTP-PEST-dependent tyrosine dephosphorylation of GIT, paxillin and FAK, as well as the GIT-dependent inhibition of Arf6 signaling to Rac (not shown). The serine/threonine phosphatase PP2A might also be recruited to paxillin to dephosphorylate S273.